Miniaturization of hiPSC-derived 3D neural cultures in stirred-tank bioreactors for parallelized preclinical assessment of rAAV
- PMID: 38737536
- PMCID: PMC11082387
- DOI: 10.3389/fbioe.2024.1379597
Miniaturization of hiPSC-derived 3D neural cultures in stirred-tank bioreactors for parallelized preclinical assessment of rAAV
Abstract
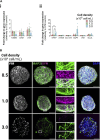
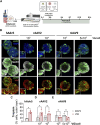
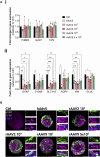
Introduction: Engineered 3D models employing human induced pluripotent stem cell (hiPSC) derivatives have the potential to recapitulate the cell diversity and structure found in the human central nervous system (CNS). Therefore, these complex cellular systems offer promising human models to address the safety and potency of advanced therapy medicinal products (ATMPs), such as gene therapies. Specifically, recombinant adeno-associated viruses (rAAVs) are currently considered highly attractive for CNS gene therapy due to their broad tropism, low toxicity, and moderate immunogenicity. To accelerate the clinical translation of rAAVs, in-depth preclinical evaluation of efficacy and safety in a human setting is primordial. The integration of hiPSC-derived CNS models in rAAV development will require, amongst other factors, robust, small-scale, high-throughput culture platforms that can feed the preclinical trials. Methods: Herein, we pioneer the miniaturization and parallelization of a 200 mL stirred-tank bioreactor-based 3D brain cell culture derived from hiPSCs. We demonstrate the applicability of the automated miniaturized Ambr® 15 Cell Culture system for the maintenance of hiPSC-derived neurospheroids (iNSpheroids), composed of neuronal and glial cells. Critical process parameters were optimized, namely, cell density and agitation mode. Results: Under optimized conditions, stable iNSpheroid cultures were attained in the microbioreactors for at least 15 days, with high cell viability and astrocytic and neuronal phenotype maintenance. This culture setup allowed the parallelization of different rAAVs, in different multiplicity of infections (MOIs), to address rAAV-host interactions at a preclinical scale. The iNSpheroids were exposed to rAAV2- and rAAV9-eGFP in the microbioreactors. Transgene expression was detected 14 days post-transduction, revealing different astrocyte/neuron tropism of the two serotypes. Discussion: We advocate that the iNSpheroid cultures in miniaturized bioreactors are reliable and reproducible screening tools for addressing rAAV transduction and tropism, compatible with preclinical demands.
Keywords: 3D (three dimensional) models; CNS modeling; gene therapy; human induced pluripotent stem cell (hiPSC); miniaturization; recombinant adeno-associated viruses (rAAVs); stem cell bioengineering; stirred-tank bioreactor.
Copyright © 2024 Gomes, Sebastião, Silva, Moura, Simão, Gomes-Alves, Alves and Brito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Perfusion Stirred-Tank Bioreactors for 3D Differentiation of Human Neural Stem Cells.Methods Mol Biol. 2016;1502:129-42. doi: 10.1007/7651_2016_333. Methods Mol Biol. 2016. PMID: 27032948
-
Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived from Induced Pluripotent Stem Cells.J Virol. 2019 Apr 17;93(9):e00111-19. doi: 10.1128/JVI.00111-19. Print 2019 May 1. J Virol. 2019. PMID: 30787148 Free PMC article.
-
Addressing neuroinflammation in human induced pluripotent stem cell-derived central nervous system neurospheroids.iScience. 2025 Aug 5;28(9):113246. doi: 10.1016/j.isci.2025.113246. eCollection 2025 Sep 19. iScience. 2025. PMID: 40894882 Free PMC article.
-
Human-induced pluripotent stem cell-derived cardiomyocytes, 3D cardiac structures, and heart-on-a-chip as tools for drug research.Pflugers Arch. 2021 Jul;473(7):1061-1085. doi: 10.1007/s00424-021-02536-z. Epub 2021 Feb 24. Pflugers Arch. 2021. PMID: 33629131 Free PMC article. Review.
-
Scalable stirred-suspension bioreactor culture of human pluripotent stem cells.Tissue Eng Part A. 2010 Feb;16(2):405-21. doi: 10.1089/ten.tea.2009.0454. Tissue Eng Part A. 2010. PMID: 19739936 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

