Evaluating the Efficacy, Safety, and Tolerability of Combination Therapy of Dapagliflozin and Linagliptin Over Dapagliflozin and Vildagliptin in Patients With Type 2 Diabetes Mellitus Inadequately Controlled With Metformin
- PMID: 38738005
- PMCID: PMC11088817
- DOI: 10.7759/cureus.58115
Evaluating the Efficacy, Safety, and Tolerability of Combination Therapy of Dapagliflozin and Linagliptin Over Dapagliflozin and Vildagliptin in Patients With Type 2 Diabetes Mellitus Inadequately Controlled With Metformin
Abstract
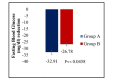
Background Type 2 diabetes mellitus (T2DM) patients commonly undergo metformin monotherapy. This study aims to compare the efficacy, safety, and tolerability of combination therapy of dapagliflozin plus linagliptin versus dapagliflozin plus vildagliptin as add-on therapy in T2DM patients inadequately controlled on metformin. Methodology This was an 18-week, multicenter, randomized, double-blind, active-controlled, parallel-group, phase III clinical study. About 236 participants were randomly assigned to receive either a fixed-dose combination of dapagliflozin 10 mg plus linagliptin 5 mg tablets or a fixed-dose combination of dapagliflozin 10 mg plus vildagliptin SR 100 mg tablets added to metformin monotherapy. The primary outcome was the mean change in hemoglobin A1c (HbA1c) from baseline to the end of week 16. The key secondary endpoints were mean change in postprandial blood glucose (PPBG), fasting blood glucose (FBG), body weight, and the proportion of participants achieving HbA1c less than 7.0%. Results The dapagliflozin/linagliptin combination therapy showed a more significant change in HbA1c from baseline to the end of 16 weeks (mean reduction: -1.59% vs. -1.25%) compared to dapagliflozin/vildagliptin (p < 0.0001). Additionally, compared to the dapagliflozin/vildagliptin group, the dapagliflozin/linagliptin group demonstrated a significant reduction in both PPBG (mean reduction: -59.99 mg/dL vs. -55.34 mg/dL) and FPG (mean reduction: -32.91 mg/dL vs. -26.78 mg/dL). A total of 18 adverse events were reported in 17 (7.20%) participants, all of which were mild and resolved completely. There were no serious adverse events. Conclusions Compared to dapagliflozin and vildagliptin combination therapy, dapagliflozin and linagliptin fixed-dose combination provided clinically significant improvements in glycemic control. Because of its effectiveness, safety, and tolerability, the fixed-dose combination of dapagliflozin and linagliptin was a better option for treating T2DM patients who had previously only received metformin monotherapy.
Keywords: dapagliflozin; glycemic control; linagliptin; metformin; type 2 diabetes mellitus; vildagliptin.
Copyright © 2024, Dharmalingam et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- IDF Diabetes Atlas. [ Dec; 2023 ]. 2021. https://diabetesatlas.org/2022-reports/ https://diabetesatlas.org/2022-reports/ - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
