Development of immediate-release formulation with reliable absorption of rivaroxaban in various meal regimes
- PMID: 38738493
- PMCID: PMC11089494
- DOI: 10.1111/cts.13820
Development of immediate-release formulation with reliable absorption of rivaroxaban in various meal regimes
Abstract
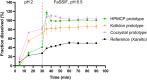
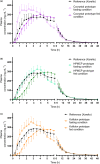
The bioavailability of rivaroxaban at the higher doses (15 and 20 mg) is considerably reduced when the drug is administered on an empty stomach. This can lead to inadequate anticoagulant effect, and therefore, it is recommended to use the higher doses at fed state. However, proper posology may represent a barrier for some patients. Therefore, the aim of this study was to evaluate innovative rivaroxaban-containing formulations designed to eliminate the food effect to ensure reliable absorption and thus to improve patient adherence with the treatment. Three prototypes (Cocrystal, HPMCP and Kollidon) with rivaroxaban were developed and their bioavailability and food effect in comparison to the reference product was tested in open label, randomized, single oral dose, crossover studies, where test products were administered under fasting and fed conditions and the reference product was administered under fed conditions. Comparable bioavailability for all tested prototypes both under fed and fasting conditions was demonstrated as the 90% confidence intervals of the geometric mean ratios for area under the concentration-time curve remained within the standard acceptance range of 80.00%-125.00%. An innovative immediate release form of rivaroxaban with no food effect on drug bioavailability has been developed, which may represent an important step toward increasing adherence, improving treatment outcome and reducing health care costs.
© 2024 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
JaBo, TK, IO, JoBe and TH are employees of Zentiva, k.s., Prague, Czech Republic, that sponsored the development program. All other authors declared no competing interests for this work.
Figures

References
-
- Kvasnicka T, Malikova I, Zenahlikova Z, et al. Rivaroxaban—metabolism, pharmacologic properties and drug interactions. Curr Drug Metab. 2017;18:636‐642. - PubMed
-
- Escolar G, Carne X, Arellano‐Rodrigo E. Dosing of rivaroxaban by indication: getting the right dose for the patient. Expert Opin Drug Metab Toxicol. 2015;11:1665‐1677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

