Body weight and composition endpoints in cancer cachexia clinical trials: Systematic Review 4 of the cachexia endpoints series
- PMID: 38738581
- PMCID: PMC11154800
- DOI: 10.1002/jcsm.13478
Body weight and composition endpoints in cancer cachexia clinical trials: Systematic Review 4 of the cachexia endpoints series
Abstract
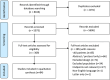
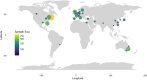
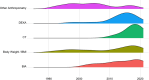
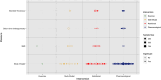
Significant variation exists in the outcomes used in cancer cachexia trials, including measures of body composition, which are often selected as primary or secondary endpoints. To date, there has been no review of the most commonly selected measures or their potential sensitivity to detect changes resulting from the interventions being examined. The aim of this systematic review is to assess the frequency and diversity of body composition measures that have been used in cancer cachexia trials. MEDLINE, Embase and Cochrane Library databases were systematically searched between January 1990 and June 2021. Eligible trials examined adults (≥18 years) who had received an intervention aiming to treat or attenuate the effects of cancer cachexia for >14 days. Trials were also of a prospective controlled design and included body weight or at least one anthropometric, bioelectrical or radiological endpoint pertaining to body composition, irrespective of the modality of intervention (e.g., pharmacological, nutritional, physical exercise and behavioural) or comparator. Trials with a sample size of <40 patients were excluded. Data extraction used Covidence software, and reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidance. This review was prospectively registered (PROSPERO: CRD42022276710). A total of 84 clinical trials, comprising 13 016 patients, were eligible for inclusion. Non-small-cell lung cancer and pancreatic cancer were studied most frequently. The majority of trial interventions were pharmacological (52%) or nutritional (34%) in nature. The most frequently reported endpoints were assessments of body weight (68 trials, n = 11 561) followed by bioimpedance analysis (BIA)-based estimates (23 trials, n = 3140). Sixteen trials (n = 3052) included dual-energy X-ray absorptiometry (DEXA)-based endpoints, and computed tomography (CT) body composition was included in eight trials (n = 841). Discrepancies were evident when comparing the efficacy of interventions using BIA-based estimates of lean tissue mass against radiological assessment modalities. Body weight, BIA and DEXA-based endpoints have been most frequently used in cancer cachexia trials. Although the optimal endpoints cannot be determined from this review, body weight, alongside measurements from radiological body composition analysis, would seem appropriate. The choice of radiological modality is likely to be dependent on the trial setting, population and intervention in question. CT and magnetic resonance imaging, which have the ability to accurately discriminate tissue types, are likely to be more sensitive and provide greater detail. Endpoints are of particular importance when aligned with the intervention's mechanism of action and/or intended patient benefit.
Keywords: body composition; cachexia; cancer cachexia; clinical trials.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
LRB, MSS, MSY, VEB, DCM, AB, TRB, OD, RDD, MTF, CG, MJH, GJ, MM, JM, IOO, IP, JS, MRS, OMV and TSS have none to declare. JA has received lecture fees from Baxter and Danone. RJES has received personal fees for consultancy from Avidity Biosciences, Actimed, Faraday and Helsinn. BJAL has received personal fees for consultancy from Artelo, Actimed, Faraday, Kyowa Kirin and Toray.
Figures

References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. - PubMed
-
- Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. - PubMed
-
- Fearon KCH. Cancer cachexia and fat–muscle physiology. N Engl J Med 2011;365:565–567. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

