Preformed Vesicle Approach to LNP Manufacturing Enhances Retinal mRNA Delivery
- PMID: 38738752
- PMCID: PMC11661498
- DOI: 10.1002/smll.202400815
Preformed Vesicle Approach to LNP Manufacturing Enhances Retinal mRNA Delivery
Abstract
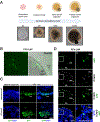
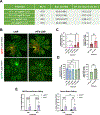
Complete encapsulation of nucleic acids by lipid-based nanoparticles (LNPs) is often thought to be one of the main prerequisites for successful nucleic acid delivery, as the lipid environment protects mRNA from degradation by external nucleases and assists in initiating delivery processes. However, delivery of mRNA via a preformed vesicle approach (PFV-LNPs) defies this precondition. Unlike traditional LNPs, PFV-LNPs are formed via a solvent-free mixing process, leading to a superficial mRNA localization. While demonstrating low encapsulation efficiency in the RiboGreen assay, PFV-LNPs improved delivery of mRNA to the retina by up to 50% compared to the LNP analogs across several benchmark formulations, suggesting the utility of this approach regardless of the lipid composition. Successful mRNA and gene editors' delivery is observed in the retinal pigment epithelium and photoreceptors and validated in mice, non-human primates, and human retinal organoids. Deploying PFV-LNPs in gene editing experiments result in a similar extent of gene editing compared to analogous LNP (up to 3% on genomic level) in the Ai9 reporter mouse model; but, remarkably, retinal tolerability is significantly improved for PFV-LNP treatment. The study findings indicate that the LNP formulation process can greatly influence mRNA transfection and gene editing outcomes, improving LNP treatment safety without sacrificing efficacy.
Keywords: IRD; gene delivery; lipid nanoparticle; mRNA; retinal degeneration.
© 2024 Wiley‐VCH GmbH.
Conflict of interest statement
Conflicts of Interest
G.S. is a co-founder of EnterX Bio and RNAvax Bio, and has an advisory role to Rare Air Inc. and Serina Tx. Y.E. has stock options and an advisory role to EnterX Bio. Other authors have no conflicts to declare.
Figures




References
-
- Russell S; Bennett J; Wellman JA; Chung DC; Yu Z-F; Tillman A; Wittes J; Pappas J; Elci O; McCague S; Cross D; Marshall KA; Walshire J; Kehoe TL; Reichert H; Davis M; Raffini L; George LA; Hudson FP; Dingfield L; Zhu X; Haller JA; Sohn EH; Mahajan VB; Pfeifer W; Weckmann M; Johnson C; Gewaily D; Drack A; Stone E; Wachtel K; Simonelli F; Leroy BP; Wright JF; High KA; Maguire AM Efficacy and Safety of Voretigene Neparvovec (AAV2-hRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. The Lancet 2017, 390 (10097), 849–860. 10.1016/S0140-6736(17)31868-8. - DOI - PMC - PubMed
-
- Hajj KA; Whitehead KA Tools for Translation: Non-Viral Materials for Therapeutic mRNA Delivery. Nat. Rev. Mater. 2017, 2 (10), 17056. 10.1038/natrevmats.2017.56. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

