Tuning Excited State Character in Iridium(III) Photosensitizers and Its Influence on TTA-UC
- PMID: 38738860
- PMCID: PMC11134496
- DOI: 10.1021/acs.inorgchem.4c01003
Tuning Excited State Character in Iridium(III) Photosensitizers and Its Influence on TTA-UC
Abstract
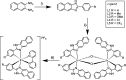
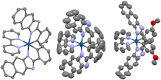
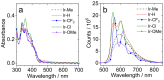
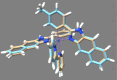
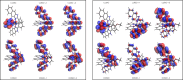
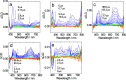
A series of mixed ligand, photoluminescent organometallic Ir(III) complexes have been synthesized to incorporate substituted 2-phenyl-1H-naphtho[2,3-d]imidazole cyclometalating ligands. The structures of three example complexes were categorically confirmed using X-ray crystallography each sharing very similar structural traits including evidence of interligand hydrogen bond contacts that account for the shielding effects observed in the 1H NMR spectra. The structural iterations of the cyclometalated ligand provide tuning of the principal electronic transitions that determine the visible absorption and emission properties of the complexes: emission can be tuned in the visible region between 550 and 610 nm and with triplet lifetimes up to 10 μs. The nature of the emitting state varies across the series of complexes, with different admixtures of ligand-centered and metal-to-ligand charge transfer triplet levels evident. Finally, the use of the complexes as photosensitizers in triplet-triplet annihilation energy upconversion (TTA-UC) was investigated in the solution state. The study showed that the complexes possessing the longest triplet lifetimes showed good viability as photosensitizers in TTA-UC. Therefore, the use of an electron-withdrawing group on the 2-phenyl-1H-naphtho[2,3-d]imidazole ligand framework can be used to rationally promote TTA-UC using this class of complex.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Lu Y.; Wang J.; McGoldrick N.; Cui X.; Zhao J.; Caverly C.; Twamley B.; Maille G. M. O.; Irwin B.; Conway-Kenny R.; et al. Iridium(III) Complexes Bearing Pyrene-Functionalized 1,10-Phenanthroline Ligands as Highly Efficient Sensitizers for Triplet–Triplet Annihilation Upconversion. Chem., Int. Ed. 2016, 55 (47), 14688–14692. 10.1002/anie.201608442. - DOI - PubMed
-
- Phillips K. A.; Stonelake T. M.; Chen K.; Hou Y.; Zhao J.; Coles S. J.; Horton P. N.; Keane S. J.; Stokes E. C.; Fallis I. A.; et al. Ligand-Tuneable, Red-Emitting Iridium(III) Complexes for Efficient Triplet–Triplet Annihilation Upconversion Performance. Chem. - Eur. J. 2018, 24, 8577.10.1002/chem.201801007. - DOI - PubMed
LinkOut - more resources
Full Text Sources

