Semaglutide and diuretic use in obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials
- PMID: 38739118
- PMCID: PMC11400859
- DOI: 10.1093/eurheartj/ehae322
Semaglutide and diuretic use in obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials
Abstract
Background and aims: In the STEP-HFpEF trial programme, treatment with semaglutide resulted in multiple beneficial effects in patients with obesity-related heart failure with preserved ejection fraction (HFpEF). Efficacy may vary according to baseline diuretic use, and semaglutide treatment could modify diuretic dose.
Methods: In this pre-specified analysis of pooled data from the STEP-HFpEF and STEP-HFpEF-DM trials (n = 1145), which randomized participants with HFpEF and body mass index ≥ 30 kg/m2 to once weekly semaglutide 2.4 mg or placebo for 52 weeks, we examined whether efficacy and safety endpoints differed by baseline diuretic use, as well as the effect of semaglutide on loop diuretic use and dose changes over the 52-week treatment period.
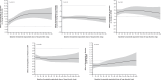
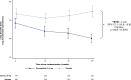
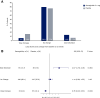
Results: At baseline, across no diuretic (n = 220), non-loop diuretic only (n = 223), and loop diuretic [<40 (n = 219), 40 (n = 309), and >40 (n = 174) mg/day furosemide equivalents] groups, there was progressively higher prevalence of hypertension and atrial fibrillation; and greater severity of obesity and heart failure. Over 52 weeks of treatment, semaglutide had a consistent beneficial effect on change in body weight across diuretic use categories (adjusted mean difference vs. placebo ranged from -8.8% [95% confidence interval (CI) -10.3, -6.3] to -6.9% [95% CI -9.1, -4.7] from no diuretics to the highest loop diuretic dose category; interaction P = .39). Kansas City Cardiomyopathy Questionnaire clinical summary score improvement was greater in patients on loop diuretics compared to those not on loop diuretics (adjusted mean difference vs. placebo: +9.3 [6.5; 12.1] vs. +4.7 points [1.3, 8.2]; P = .042). Semaglutide had consistent beneficial effects on all secondary efficacy endpoints (including 6 min walk distance) across diuretic subgroups (interaction P = .24-.92). Safety also favoured semaglutide vs. placebo across the diuretic subgroups. From baseline to 52 weeks, loop diuretic dose decreased by 17% in the semaglutide group vs. a 2.4% increase in the placebo group (P < .0001). Semaglutide (vs. placebo) was more likely to result in loop diuretic dose reduction (odds ratio [OR] 2.67 [95% CI 1.70, 4.18]) and less likely dose increase (OR 0.35 [95% CI 0.23, 0.53]; P < .001 for both) from baseline to 52 weeks.
Conclusions: In patients with obesity-related HFpEF, semaglutide improved heart failure-related symptoms and physical limitations across diuretic use subgroups, with more pronounced benefits among patients receiving loop diuretics at baseline. Reductions in weight and improvements in exercise function with semaglutide vs. placebo were consistent in all diuretic use categories. Semaglutide also led to a reduction in loop diuretic use and dose between baseline and 52 weeks.
Clinical trial registration: NCT04788511 and NCT04916470.
Keywords: Clinical trial; Glucagon-like peptide-1 receptor agonist; Heart failure with preserved ejection fraction; Loop diuretics; Obesity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Pius R, Odukudu GO, Olorundare I, Makanjuola DI, Komolafe R, Njoku C, et al. . A narrative review on the efficacy and safety of loop diuretics in patients with heart failure with reduced ejection fraction and preserved ejection fraction. Cureus 2023;15:e45794. 10.7759/cureus.45794 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

