CCL4 contributes to aging related angiogenic insufficiency through activating oxidative stress and endothelial inflammation
- PMID: 38739303
- PMCID: PMC11303582
- DOI: 10.1007/s10456-024-09922-y
CCL4 contributes to aging related angiogenic insufficiency through activating oxidative stress and endothelial inflammation
Erratum in
-
Correction: CCL4 contributes to aging related angiogenic insufficiency through activating oxidative stress and endothelial inflammation.Angiogenesis. 2025 Feb 28;28(2):18. doi: 10.1007/s10456-024-09964-2. Angiogenesis. 2025. PMID: 40019597 Free PMC article. No abstract available.
Abstract
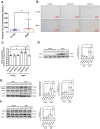
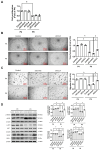
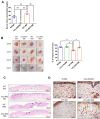
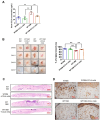
Aging is a natural process associated with chronic inflammation in the development of vascular dysfunction. We hypothesized that chemokine C-C motif ligands 4 (CCL4) might play a vital role in aging-related vascular dysfunction. Circulating CCL4 was up-regulated in elderly subjects and in aged animals. CCL4 inhibition reduced generation of reactive oxygen species (ROS), attenuated inflammation, and restored cell functions in endothelial progenitor cells from elderly subjects and in aged human aortic endothelial cells. CCL4 promoted cell aging, with impaired cell functioning, by activating ROS production and inflammation. CCL4 knockout mice and therapeutic administration of anti-CCL4 neutralizing antibodies exhibited vascular and dermal anti-aging effects, with improved wound healing, via the down-regulation of inflammatory proteins and the activation of angiogenic proteins. Altogether, our findings suggested that CCL4 may contribute to aging-related vascular dysfunction via activating oxidative stress and endothelial inflammation. CCL4 may be a potential therapeutic target for vascular protections during aging.
Keywords: Aging; CCL4; Inflammation; Oxidative stress; Vascular dysfunction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures












References
-
- Bachmann MC, Bellalta S, Basoalto R, Gómez-Valenzuela F, Jalil Y, Lépez M, Matamoros A, von Bernhardi R (2020) The challenge by multiple environmental and biological factors induce inflammation in aging: their role in the promotion of chronic disease. Front Immunol 11:570083. 10.3389/fimmu.2020.570083 - PMC - PubMed
-
- Finkel T (2015) The metabolic regulation of aging. Nat Med 21(12):1416–1423. 10.1038/nm.3998 - PubMed
-
- Lodi PJ, Garrett DS, Kuszewski J, Tsang ML, Weatherbee JA, Leonard WJ, Gronenborn AM, Clore GM (1994) High-resolution solution structure of the beta chemokine hMIP-1 beta by multidimensional NMR. Science 263(5154):1762–1767. 10.1126/science.8134838 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

