Contribution of mechanoreceptors to spinal cord injury-induced mechanical allodynia
- PMID: 38739766
- PMCID: PMC11090032
- DOI: 10.1097/j.pain.0000000000003139
Contribution of mechanoreceptors to spinal cord injury-induced mechanical allodynia
Abstract
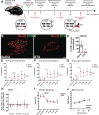
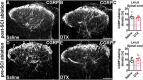
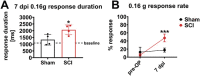
Evidence from previous studies supports the concept that spinal cord injury (SCI)-induced neuropathic pain (NP) has its neural roots in the peripheral nervous system. There is uncertainty about how and to which degree mechanoreceptors contribute. Sensorimotor activation-based interventions (eg, treadmill training) have been shown to reduce NP after experimental SCI, suggesting transmission of pain-alleviating signals through mechanoreceptors. The aim of the present study was to understand the contribution of mechanoreceptors with respect to mechanical allodynia in a moderate mouse contusion SCI model. After genetic ablation of tropomyosin receptor kinase B expressing mechanoreceptors before SCI, mechanical allodynia was reduced. The identical genetic ablation after SCI did not yield any change in pain behavior. Peptidergic nociceptor sprouting into lamina III/IV below injury level as a consequence of SCI was not altered by either mechanoreceptor ablation. However, skin-nerve preparations of contusion SCI mice 7 days after injury yielded hyperexcitability in nociceptors, not in mechanoreceptors, which makes a substantial direct contribution of mechanoreceptors to NP maintenance unlikely. Complementing animal data, quantitative sensory testing in human SCI subjects indicated reduced mechanical pain thresholds, whereas the mechanical detection threshold was not altered. Taken together, early mechanoreceptor ablation modulates pain behavior, most likely through indirect mechanisms. Hyperexcitable nociceptors seem to be the main drivers of SCI-induced NP. Future studies need to focus on injury-derived factors triggering early-onset nociceptor hyperexcitability, which could serve as targets for more effective therapeutic interventions.
Trial registration: ClinicalTrials.gov NCT01571531.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, Lechner SG. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron 2017;93:179–93. - PubMed
-
- Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res 2011;1370:129–35. - PubMed
-
- Baumgärtner U, Magerl W, Klein T, Hopf HC, Treede RD. Neurogenic hyperalgesia versus painful hypoalgesia: two distinct mechanisms of neuropathic pain. PAIN 2002;96:141–51. - PubMed
-
- Bavencoffe A, Li Y, Wu Z, Yang Q, Herrera J, Kennedy EJ, Walters ET, Dessauer CW. Persistent electrical activity in primary nociceptors after spinal cord injury is maintained by scaffolded adenylyl cyclase and protein kinase A and is associated with altered adenylyl cyclase regulation. J Neurosci 2016;36:1660–8. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

