Pattern-Recognition Receptors and Immunometabolic Reprogramming: What We Know and What to Explore
- PMID: 38740018
- PMCID: PMC11250681
- DOI: 10.1159/000539278
Pattern-Recognition Receptors and Immunometabolic Reprogramming: What We Know and What to Explore
Abstract
Background: Evolutionarily, immune response is a complex mechanism that protects the host from internal and external threats. Pattern-recognition receptors (PRRs) recognize MAMPs, PAMPs, and DAMPs to initiate a protective pro-inflammatory immune response. PRRs are expressed on the cell membranes by TLR1, 2, 4, and 6 and in the cytosolic organelles by TLR3, 7, 8, and 9, NLRs, ALRs, and cGLRs. We know their downstream signaling pathways controlling immunoregulatory and pro-inflammatory immune response. However, the impact of PRRs on metabolic control of immune cells to control their pro- and anti-inflammatory activity has not been discussed extensively.
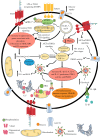
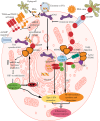
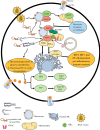
Summary: Immune cell metabolism or immunometabolism critically determines immune cells' pro-inflammatory phenotype and function. The current article discusses immunometabolic reprogramming (IR) upon activation of different PRRs, such as TLRs, NLRs, cGLRs, and RLRs. The duration and type of PRR activated, species studied, and location of immune cells to specific organ are critical factors to determine the IR-induced immune response.
Key message: The work herein describes IR upon TLR, NLR, cGLR, and RLR activation. Understanding IR upon activating different PRRs is critical for designing better immune cell-specific immunotherapeutics and immunomodulators targeting inflammation and inflammatory diseases.
Keywords: Glycolysis; Immunometabolism; Infection; Inflammation; NOD-like receptors; Oxidative phosphorylation; Retinoic acid-inducible gene-1-like receptors; Toll-like receptors; cGLRs.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Authors declare no competing interest.
Figures
References
-
- Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–67. - PubMed
-
- Michels N, van Aart C, Morisse J, Mullee A, Huybrechts I. Chronic inflammation towards cancer incidence: a systematic review and meta-analysis of epidemiological studies. Crit Rev Oncol Hematol. 2021;157:103177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

