Quality of Essential Medicines from Different Sources in Enugu and Anambra, Nigeria
- PMID: 38740019
- PMCID: PMC11229646
- DOI: 10.4269/ajtmh.23-0837
Quality of Essential Medicines from Different Sources in Enugu and Anambra, Nigeria
Abstract
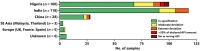
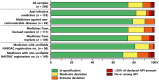
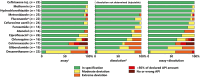
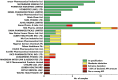
This study investigated the quality of 13 essential medicines in the states of Enugu and Anambra, Nigeria. A total of 260 samples were purchased from licensed pharmaceutical manufacturers and wholesalers and from vendors in pharmaceutical markets with unclear licensing status. Samples were analyzed for identity, content, and dissolution according to the United States Pharmacopeia (USP) 42 monographs. Forty-five samples of this study could be examined for authenticity with the Mobile Authentication Service scheme of the Nigerian National Agency for Food and Drug Administration and Control. Out of all samples, 25.4% did not comply with the USP 42 specifications. Strikingly, 21 out of 22 dexamethasone tablet samples (95%) were out of specification (OOS). Nine out of 19 glibenclamide samples (47%) failed dissolution testing, and 7 out of 17 cotrimoxazole samples (41%) failed assay testing. Medicines against noncommunicable diseases showed a slightly higher percentage of OOS samples than anti-infectives (21.2% versus 17.6%). The rates of OOS samples were similar in medicines stated to be produced in Nigeria, India, and China but were very different between individual manufacturers from each of these countries of origin. Therefore, prequalification of products, manufacturers, and suppliers are very important for quality assurance in medicine procurement. Unexpectedly, the total proportions of OOS samples were similar from licensed vendors (25.2%) and from markets (25.5%). Four samples (1.5%), all collected in markets, were clearly falsified and did not contain the declared active pharmaceutical ingredients. The proportion of falsified medicines was found to be lower than frequently reported in the media for Nigeria.
Conflict of interest statement
Disclosure: The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures



References
-
- United Nations DESA , 2017. Sustainable Development Goal 3: Ensure Healthy Lives and Promote Well-Being for All at All Ages—Targets and Indicators. Available at: https://sdgs.un.org/goals/goal3#targets_and_indicators. Accessed November 15, 2023.
-
- World Health Organization , 2017. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. Available at: https://www.who.int/publications/i/item/9789241513432. Accessed November 15, 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

