Extended pleurectomy decortication and chemotherapy versus chemotherapy alone for pleural mesothelioma (MARS 2): a phase 3 randomised controlled trial
- PMID: 38740044
- PMCID: PMC11136673
- DOI: 10.1016/S2213-2600(24)00119-X
Extended pleurectomy decortication and chemotherapy versus chemotherapy alone for pleural mesothelioma (MARS 2): a phase 3 randomised controlled trial
Abstract
Background: Extended pleurectomy decortication for complete macroscopic resection for pleural mesothelioma has never been evaluated in a randomised trial. The aim of this study was to compare outcomes after extended pleurectomy decortication plus chemotherapy versus chemotherapy alone.
Methods: MARS 2 was a phase 3, national, multicentre, open-label, parallel two-group, pragmatic, superiority randomised controlled trial conducted in the UK. The trial took place across 26 hospitals (21 recruiting only, one surgical only, and four recruiting and surgical). Following two cycles of chemotherapy, eligible participants with pleural mesothelioma were randomly assigned (1:1) to surgery and chemotherapy or chemotherapy alone using a secure web-based system. Individuals aged 16 years or older with resectable pleural mesothelioma and adequate organ and lung function were eligible for inclusion. Participants in the chemotherapy only group received two to four further cycles of chemotherapy, and participants in the surgery and chemotherapy group received pleurectomy decortication or extended pleurectomy decortication, followed by two to four further cycles of chemotherapy. It was not possible to mask allocation because the intervention was a major surgical procedure. The primary outcome was overall survival, defined as time from randomisation to death from any cause. Analyses were done on the intention-to-treat population for all outcomes, unless specified. This study is registered with ClinicalTrials.gov, NCT02040272, and is closed to new participants.
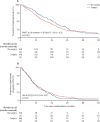
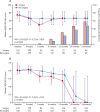
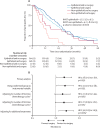
Findings: Between June 19, 2015, and Jan 21, 2021, of 1030 assessed for eligibility, 335 participants were randomly assigned (169 to surgery and chemotherapy, and 166 to chemotherapy alone). 291 (87%) participants were men and 44 (13%) women, and 288 (86%) were diagnosed with epithelioid mesothelioma. At a median follow-up of 22·4 months (IQR 11·3-30·8), median survival was shorter in the surgery and chemotherapy group (19·3 months [IQR 10·0-33·7]) than in the chemotherapy alone group (24·8 months [IQR 12·6-37·4]), and the difference in restricted mean survival time at 2 years was -1·9 months (95% CI -3·4 to -0·3, p=0·019). There were 318 serious adverse events (grade ≥3) in the surgery group and 169 in the chemotherapy group (incidence rate ratio 3·6 [95% CI 2·3 to 5·5], p<0·0001), with increased incidence of cardiac (30 vs 12; 3·01 [1·13 to 8·02]) and respiratory (84 vs 34; 2·62 [1·58 to 4·33]) disorders, infection (124 vs 53; 2·13 [1·36 to 3·33]), and additional surgical or medical procedures (15 vs eight; 2·41 [1·04 to 5·57]) in the surgery group.
Interpretation: Extended pleurectomy decortication was associated with worse survival to 2 years, and more serious adverse events for individuals with resectable pleural mesothelioma, compared with chemotherapy alone.
Funding: National Institute for Health and Care Research (NIHR) Health Technology Assessment programme (15/188/31), Cancer Research UK Feasibility Studies Project Grant (A15895).
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests EL reports grants from Boehringer Ingelheim, Medela, Johnson & Johnson/Ethicon, Covidien/Medtronic, Guardant Health, Takeda, Lilly Oncology, and Bayer, paid to his institution, his company, or personally; consulting fees from BeiGene, Roche, and BMS; honoraria from Medela; two patents (P52435GB and P57988GB) issued to Imperial Innovations; and being founder of My Cancer Companion, Healthcare Companion. SP reports consulting fees from AnHeart Therapeutics, Amgen, AstraZeneca, Bayer, Blueprint, BMS, Boehringer Ingelheim, Ellipses, EQRx, Daiichi Sankyo, GSK, Guardant Health, IO Biotech, Janssen, Lilly, Merck Serono, Mirati, MSD, Novocure, Novartis, PharmaMar, Roche, Takeda, Pfizer, Pierre Fabre, and Turning Point Therapeutics; honoraria from AstraZeneca, Bayer, Guardant Health, Janssen, Merck Serono, Roche, and Takeda; fees for expert testimony from Roche and Merck Serono; support for meeting attendance from Janssen, Roche, and Gilead; and being a member of the British Thoracic Oncology Group, ALK Positive UK, Lung Cancer Europe, Ruth Strauss Foundation, Mesothelioma Applied Research Foundation, and ETOP-IBCSG Partners Foundation Board. NT reports honoraria from BMS. DF reports grants from Astex Therapeutics, Boehringer Ingelheim, Bayer Oncology, Bergen Bio, GSK, MSD, Owkin, Roche, and RS Oncology paid to his institution; consulting fees from MSD, Cambridge Clinical Laboratories, and RS Oncology; honoraria from BMS, BI, MSD, Ikena, and Owkin; and meeting support from RS Oncology and MSD, all paid to Thoracic Oncology Services where he is director. RCR reports research funding from Cancer Research UK, Asthma and Lung UK, June Hancock Mesothelioma Research Fund, Mick Knighton Mesothelioma Research Fund, and Mesobank; and participation on an advisory board for the UK Lung Cancer Coalition. PT reports honoraria from AstraZeneca. LC-S reports support for meeting attendance from Lilly. RC reports honoraria from AstraZeneca, MSD, Takeda, Janssen, Roche, and GSK; support for meeting attendance from Takeda and Janssen; participation on advisory boards for GSK, Takeda, Janssen, Pharmamar, and Amgen; and stock options with TCC and Supportive Care UK. YS reports honoraria from Amgen, AstraZeneca, AbbVie, BMS, MSD, Lilly, Roche, and Takeda; and support for meeting attendance from Takeda and Roche. ZT reports honoraria from AstraZeneca. RS reports honoraria and support for meeting attendance from Lilly and BMS. EF reports membership of the National Lung Cancer and Mesothelioma Clinical Experts Group and Northern Cancer Alliance Targeted Lung Health Check Clinical Lead. All other authors declare no competing interests.
Figures

References
-
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. - PubMed
-
- National Comprehensive Cancer Network NCCN Guidelines: Mesothelioma: pleural. 2023. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1512
-
- Opitz I, Scherpereel A, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2020;58:1–24. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

