Suppression of hepatic ChREBP⍺-CYP2C50 axis-driven fatty acid oxidation sensitizes mice to diet-induced MASLD/MASH
- PMID: 38740087
- PMCID: PMC11145360
- DOI: 10.1016/j.molmet.2024.101957
Suppression of hepatic ChREBP⍺-CYP2C50 axis-driven fatty acid oxidation sensitizes mice to diet-induced MASLD/MASH
Abstract
Objectives: Compromised hepatic fatty acid oxidation (FAO) has been observed in human MASH patients and animal models of MASLD/MASH. It remains poorly understood how and when the hepatic FAO pathway is suppressed during the progression of MASLD towards MASH. Hepatic ChREBP⍺ is a classical lipogenic transcription factor that responds to the intake of dietary sugars.
Methods: We examined its role in regulating hepatocyte fatty acid oxidation (FAO) and the impact of hepatic Chrebpa deficiency on sensitivity to diet-induced MASLD/MASH in mice.
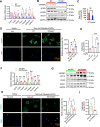
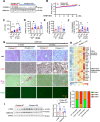
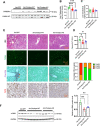
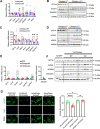
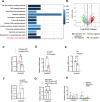
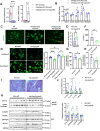
Results: We discovered that hepatocyte ChREBP⍺ is both necessary and sufficient to maintain FAO in a cell-autonomous manner independently of its DNA-binding activity. Supplementation of synthetic PPAR⍺/δ agonist is sufficient to restore FAO in Chrebp-/- primary mouse hepatocytes. Hepatic ChREBP⍺ was decreased in mouse models of diet-induced MAFSLD/MASH and in patients with MASH. Hepatocyte-specific Chrebp⍺ knockout impaired FAO, aggravated liver steatosis and inflammation, leading to early-onset fibrosis in response to diet-induced MASH. Conversely, liver overexpression of ChREBP⍺-WT or its non-lipogenic mutant enhanced FAO, reduced lipid deposition, and alleviated liver injury, inflammation, and fibrosis. RNA-seq analysis identified the CYP450 epoxygenase (CYP2C50) pathway of arachidonic acid metabolism as a novel target of ChREBP⍺. Over-expression of CYP2C50 partially restores hepatic FAO in primary hepatocytes with Chrebp⍺ deficiency and attenuates preexisting MASH in the livers of hepatocyte-specific Chrebp⍺-deleted mice.
Conclusions: Our findings support the protective role of hepatocyte ChREBPa against diet-induced MASLD/MASH in mouse models in part via promoting CYP2C50-driven FAO.
Keywords: Carbohydrate-response element binding protein (ChREBP); Fatty acid oxidation; Lipid metabolism; Metabolic-Associated Steatohepatitis.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest We declare there is no conflict interest for this manuscript.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

