A cell-free nutrient-supplemented perfusate allows four-day ex vivo metabolic preservation of human kidneys
- PMID: 38740760
- PMCID: PMC11091145
- DOI: 10.1038/s41467-024-47106-w
A cell-free nutrient-supplemented perfusate allows four-day ex vivo metabolic preservation of human kidneys
Abstract
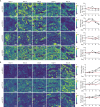
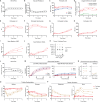
The growing disparity between the demand for transplants and the available donor supply, coupled with an aging donor population and increasing prevalence of chronic diseases, highlights the urgent need for the development of platforms enabling reconditioning, repair, and regeneration of deceased donor organs. This necessitates the ability to preserve metabolically active kidneys ex vivo for days. However, current kidney normothermic machine perfusion (NMP) approaches allow metabolic preservation only for hours. Here we show that human kidneys discarded for transplantation can be preserved in a metabolically active state up to 4 days when perfused with a cell-free perfusate supplemented with TCA cycle intermediates at subnormothermia (25 °C). Using spatially resolved isotope tracing we demonstrate preserved metabolic fluxes in the kidney microenvironment up to Day 4 of perfusion. Beyond Day 4, significant changes were observed in renal cell populations through spatial lipidomics, and increases in injury markers such as LDH, NGAL and oxidized lipids. Finally, we demonstrate that perfused kidneys maintain functional parameters up to Day 4. Collectively, these findings provide evidence that this approach enables metabolic and functional preservation of human kidneys over multiple days, establishing a solid foundation for future clinical investigations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

