Amyloid-beta antibody binding to cerebral amyloid angiopathy fibrils and risk for amyloid-related imaging abnormalities
- PMID: 38740836
- PMCID: PMC11091209
- DOI: 10.1038/s41598-024-61691-2
Amyloid-beta antibody binding to cerebral amyloid angiopathy fibrils and risk for amyloid-related imaging abnormalities
Abstract
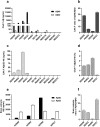
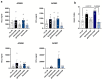
Therapeutic antibodies have been developed to target amyloid-beta (Aβ), and some of these slow the progression of Alzheimer's disease (AD). However, they can also cause adverse events known as amyloid-related imaging abnormalities with edema (ARIA-E). We investigated therapeutic Aβ antibody binding to cerebral amyloid angiopathy (CAA) fibrils isolated from human leptomeningeal tissue to study whether this related to the ARIA-E frequencies previously reported by clinical trials. The binding of Aβ antibodies to CAA Aβ fibrils was evaluated in vitro using immunoprecipitation, surface plasmon resonance, and direct binding assay. Marked differences in Aβ antibody binding to CAA fibrils were observed. Solanezumab and crenezumab showed negligible CAA fibril binding and these antibodies have no reported ARIA-E cases. Lecanemab showed a low binding to CAA fibrils, consistent with its relatively low ARIA-E frequency of 12.6%, while aducanumab, bapineuzumab, and gantenerumab all showed higher binding to CAA fibrils and substantially higher ARIA-E frequencies (25-35%). An ARIA-E frequency of 24% was reported for donanemab, and its binding to CAA fibrils correlated with the amount of pyroglutamate-modified Aβ present. The findings of this study support the proposal that Aβ antibody-CAA interactions may relate to the ARIA-E frequency observed in patients treated with Aβ-based immunotherapies.
Keywords: ARIA; Alzheimer’s disease; Amyloid; CAA; Immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
LS, MJ, EG, PN, NF, OZ, AR, AS, EB, GD, GO, LL, and CM are employees and shareholders of BioArctic. LL is co‐founder and board member of BioArctic.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

