Propionate prevents loss of the PDIM virulence lipid in Mycobacterium tuberculosis
- PMID: 38740932
- PMCID: PMC11253637
- DOI: 10.1038/s41564-024-01697-8
Propionate prevents loss of the PDIM virulence lipid in Mycobacterium tuberculosis
Abstract
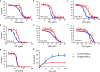
Phthiocerol dimycocerosate (PDIM) is an essential virulence lipid of Mycobacterium tuberculosis. In vitro culturing rapidly selects for spontaneous PDIM-negative mutants that have attenuated virulence and increased cell wall permeability, thus impacting the relevance of experimental findings. PDIM loss can also reduce the efficacy of the BCG Pasteur vaccine. Here we show that vancomycin susceptibility can rapidly screen for M. tuberculosis PDIM production. We find that metabolic deficiency of methylmalonyl-CoA impedes the growth of PDIM-producing bacilli, selecting for PDIM-negative variants. Supplementation with odd-chain fatty acids, cholesterol or vitamin B12 restores PDIM-positive bacterial growth. Specifically, we show that propionate supplementation enhances PDIM-producing bacterial growth and selects against PDIM-negative mutants, analogous to in vivo conditions. Our study provides a simple approach to screen for and maintain PDIM production, and reveals how discrepancies between the host and in vitro nutrient environments can attenuate bacterial pathogenicity.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
C.V.M. and M.B. are inventors on a pending patent related to this work (U.S. Patent Application No. 63/527,831, filed 20 July 2023). The authors declare that they have no other competing interests. The remaining authors declare no competing interests.
Figures





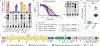

Update of
-
The PDIM paradox of Mycobacterium tuberculosis: new solutions to a persistent problem.bioRxiv [Preprint]. 2023 Oct 16:2023.10.16.562559. doi: 10.1101/2023.10.16.562559. bioRxiv. 2023. Update in: Nat Microbiol. 2024 Jun;9(6):1607-1618. doi: 10.1038/s41564-024-01697-8. PMID: 37905120 Free PMC article. Updated. Preprint.
References
-
- Daffe M & Laneelle MA Distribution of phthiocerol diester, phenolic mycosides and related compounds in mycobacteria. J Gen Microbiol 134, 2049–2055 (1988). - PubMed
-
- Rens C, Chao JD, Sexton DL, Tocheva EI & Av-Gay Y Roles for phthiocerol dimycocerosate lipids in Mycobacterium tuberculosis pathogenesis. Microbiology (Reading) 167 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

