Mitochondrial function and gastrointestinal diseases
- PMID: 38740978
- PMCID: PMC12036329
- DOI: 10.1038/s41575-024-00931-2
Mitochondrial function and gastrointestinal diseases
Abstract
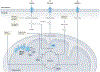
Mitochondria are dynamic organelles that function in cellular energy metabolism, intracellular and extracellular signalling, cellular fate and stress responses. Mitochondria of the intestinal epithelium, the cellular interface between self and enteric microbiota, have emerged as crucial in intestinal health. Mitochondrial dysfunction occurs in gastrointestinal diseases, including inflammatory bowel diseases and colorectal cancer. In this Review, we provide an overview of the current understanding of intestinal epithelial cell mitochondrial metabolism, function and signalling to affect tissue homeostasis, including gut microbiota composition. We also discuss mitochondrial-targeted therapeutics for inflammatory bowel diseases and colorectal cancer and the evolving concept of mitochondrial impairment as a consequence versus initiator of the disease.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

