Intraperitoneal versus intranasal administration of lipopolysaccharide in causing sepsis severity in a murine model: a preliminary comparison
- PMID: 38741131
- PMCID: PMC11089766
- DOI: 10.1186/s42826-024-00205-7
Intraperitoneal versus intranasal administration of lipopolysaccharide in causing sepsis severity in a murine model: a preliminary comparison
Abstract
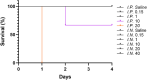
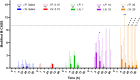
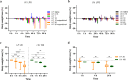
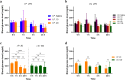
Community-acquired respiratory infection is the commonest cause of sepsis presenting to emergency departments. Yet current experimental animal models simulate peritoneal sepsis with intraperitoneal (I.P.) injection of lipopolysaccharide (LPS) as the predominant route. We aimed to compare the progression of organ injury between I.P. LPS and intranasal (I.N.) LPS in order to establish a better endotoxemia murine model of respiratory sepsis. Eight weeks old male BALB/c mice received LPS-Escherichia coli doses at 0.15, 1, 10, 20, 40 and 100 mg per kg body weight (e.g. LPS-10 is a dose of 10 mg/kg body weight). Disease severity was monitored by a modified Mouse Clinical Assessment Score for Sepsis (M-CASS; range 0-21). A M-CASS score ≥ 10 or a weight reduction of ≥ 20%, was used as a criterion for euthanasia. The primary outcome was the survival rate (either no death or no need for euthanasia). The progression of disease was specified as M-CASS, body weight, blood glucose, histopathological changes to lung, liver, spleen, kidney, brain and heart tissues. Survival rate in I.P. LPS-20 mice was 0% (2/3 died; 1/3 euthanized with M-CASS > 10) at 24 h. Survival rate in all doses of I.N. LPS was 100% (20/20; 3-4 per group) at 96 h. 24 h mean M-CASS post-I.P. LPS-10 was 6.4/21 significantly higher than I.N. LPS-10 of 1.7/21 (Unpaired t test, P < 0.05). Organ injury was present at 96 h in the I.P. LPS-10 group: lung (3/3; 100%), spleen (3/3; 100%) and liver (1/3; 33%). At 24 h in the I.P. LPS-20 group, kidney injury was observed in the euthanized mouse. At 96 h in the post-I.N. LPS-20 group, only lung injury was observed in 2/3 (67%) mice (Kruskal-Wallis test with Dunn's, P < 0.01). At 24 h in the post-I.N. LPS-100 group all (4/4) mice had evidence of lung injury. Variable doses of I.N. LPS in mice produced lung injury but did not produce sepsis. Higher doses of I.P. LPS induced multi-organ injury but not respiratory sepsis. Lethal models of respiratory virus, e.g., influenza A, might provide alternative avenues that can be explored in future research.
Keywords: Histology; Intranasal; Intraperitoneal; LPS; Organ injury; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
Similar articles
-
Time- and dose-dependent severity of lung injury in a rat model of sepsis.Rom J Morphol Embryol. 2015;56(4):1329-37. Rom J Morphol Embryol. 2015. PMID: 26743278
-
Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis.Intensive Care Med Exp. 2018 Jul 27;6(1):20. doi: 10.1186/s40635-018-0184-3. Intensive Care Med Exp. 2018. PMID: 30054760 Free PMC article.
-
A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation.J Immunol Methods. 1997 Mar 10;202(1):49-57. doi: 10.1016/s0022-1759(96)00236-0. J Immunol Methods. 1997. PMID: 9075771
-
Therapeutic benefits of the group B3 vitamin nicotinamide in mice with lethal endotoxemia and polymicrobial sepsis.Pharmacol Res. 2012 Mar;65(3):328-37. doi: 10.1016/j.phrs.2011.11.014. Epub 2011 Dec 1. Pharmacol Res. 2012. PMID: 22154801
-
Kallistatin treatment attenuates lethality and organ injury in mouse models of established sepsis.Crit Care. 2015 May 1;19(1):200. doi: 10.1186/s13054-015-0919-4. Crit Care. 2015. PMID: 25930108 Free PMC article.
Cited by
-
Nebulized Lipopolysaccharide Causes Delayed Cortical Neuroinflammation in a Murine Model of Acute Lung Injury.Int J Mol Sci. 2024 Sep 20;25(18):10117. doi: 10.3390/ijms251810117. Int J Mol Sci. 2024. PMID: 39337602 Free PMC article.
-
Use of H1N1 strain A/PR/8/34 influenza to build a mouse model of viral respiratory sepsis.Lab Anim Res. 2025 Jun 4;41(1):16. doi: 10.1186/s42826-025-00248-4. Lab Anim Res. 2025. PMID: 40468456 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

