Analysis of the components of Mycobacterium tuberculosis heat-resistant antigen (Mtb-HAg) and its regulation of γδ T-cell function
- PMID: 38741147
- PMCID: PMC11089708
- DOI: 10.1186/s11658-024-00585-7
Analysis of the components of Mycobacterium tuberculosis heat-resistant antigen (Mtb-HAg) and its regulation of γδ T-cell function
Abstract
Background: Mycobacterium tuberculosis heat-resistant antigen (Mtb-HAg) is a peptide antigen released from the mycobacterial cytoplasm into the supernatant of Mycobacterium tuberculosis (Mtb) attenuated H37Ra strain after autoclaving at 121 °C for 20 min. Mtb-HAg can specifically induce γδ T-cell proliferation in vitro. However, the exact composition of Mtb-HAg and the protein antigens that are responsible for its function are currently unknown.
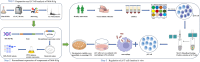
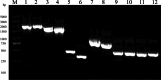
Methods: Mtb-HAg extracted from the Mtb H37Ra strain was subjected to LC‒MS mass spectrometry. Twelve of the identified protein fractions were recombinantly expressed in Escherichia coli by genetic engineering technology using pET-28a as a plasmid and purified by Ni-NTA agarose resin to stimulate peripheral blood mononuclear cells (PBMCs) from different healthy individuals. The proliferation of γδ T cells and major γδ T-cell subset types as well as the production of TNF-α and IFN-γ were determined by flow cytometry. Their proliferating γδ T cells were isolated and purified using MACS separation columns, and Mtb H37Ra-infected THP-1 was co-cultured with isolated and purified γδ T cells to quantify Mycobacterium viability by counting CFUs.
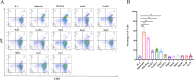
Results: In this study, Mtb-HAg from the attenuated Mtb H37Ra strain was analysed by LC‒MS mass spectrometry, and a total of 564 proteins were identified. Analysis of the identified protein fractions revealed that the major protein components included heat shock proteins and Mtb-specific antigenic proteins. Recombinant expression of 10 of these proteins in by Escherichia coli genetic engineering technology was used to successfully stimulate PBMCs from different healthy individuals, but 2 of the proteins, EsxJ and EsxA, were not expressed. Flow cytometry results showed that, compared with the IL-2 control, HspX, GroEL1, and GroES specifically induced γδ T-cell expansion, with Vγ2δ2 T cells as the main subset, and the secretion of the antimicrobial cytokines TNF-α and IFN-γ. In contrast, HtpG, DnaK, GroEL2, HbhA, Mpt63, EsxB, and EsxN were unable to promote γδ T-cell proliferation and the secretion of TNF-α and IFN-γ. None of the above recombinant proteins were able to induce the secretion of TNF-α and IFN-γ by αβ T cells. In addition, TNF-α, IFN-γ-producing γδ T cells inhibit the growth of intracellular Mtb.
Conclusion: Activated γδ T cells induced by Mtb-HAg components HspX, GroES, GroEL1 to produce TNF-α, IFN-γ modulate macrophages to inhibit intracellular Mtb growth. These data lay the foundation for subsequent studies on the mechanism by which Mtb-HAg induces γδ T-cell proliferation in vitro, as well as the development of preventive and therapeutic vaccines and rapid diagnostic reagents.
Keywords: Mycobacterium tuberculosis heat resistant antigen (Mtb-HAg); IFN-γ; TNF-α; γδ T cell.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- 1908085MH252/Anhui Provincial Natural Science Foundation
- 2008085QH405/Science Fund for Distinguished Young Scholars of Anhui Province
- by51201309/Ten Thousand Talent Plans for Young Top-notch Talents of Yunnan Province
- Byycx22013/Vicerrectoría de Investigación y Estudios de Posgrado, Benemérita Universidad Autónoma de Puebla
- Byycx23078/Postgraduate Scientific Research Innovation Program of Bengbu Medical College
LinkOut - more resources
Full Text Sources
Miscellaneous

