Management of Colorectal Neoplasia in IBD Patients: Current Practice and Future Perspectives
- PMID: 38741227
- PMCID: PMC11479698
- DOI: 10.1093/ecco-jcc/jjae071
Management of Colorectal Neoplasia in IBD Patients: Current Practice and Future Perspectives
Abstract
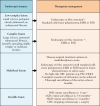
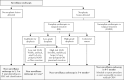
Inflammatory bowel disease [IBD] patients are at increased risk of developing colorectal neoplasia [CRN]. In this review, we aim to provide an up-to-date overview and future perspectives on CRN management in IBD. Advances in endoscopic surveillance and resection techniques have resulted in a shift towards endoscopic management of neoplastic lesions in place of surgery. Endoscopic treatment is recommended for all CRN if complete resection is feasible. Standard [cold snare] polypectomy, endoscopic mucosal resection and endoscopic submucosal dissection should be performed depending on lesion complexity [size, delineation, morphology, surface architecture, submucosal fibrosis/invasion] to maximise the likelihood of complete resection. If complete resection is not feasible, surgical treatment options should be discussed by a multidisciplinary team. Whereas [sub]total and proctocolectomy play an important role in management of endoscopically unresectable CRN, partial colectomy may be considered in a subgroup of patients in endoscopic remission with limited disease extent without other CRN risk factors. High synchronous and metachronous CRN rates warrant careful mucosal visualisation with shortened intervals for at least 5 years after treatment of CRN.
Keywords: Crohn’s disease; Inflammatory bowel disease; colorectal cancer; colorectal neoplasia; surveillance; ulcerative colitis.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
The authors state no conflict of interest. MG has served as a speaker for Abbvie. TB acted as a speaker or adviser for Abbvie, Alimentiv, Amgen, Bristol-Myers-Squibb, Eli Lilly, Ferring, Fresenius Kabi, Gilead, Janssen, Merck, Pentax, Pfizer, Roche, Sandoz, Sanofi, Takeda, Viatris. LD has served on advisory boards or as speaker for Abbvie, Janssen, Sandoz, Galapagos and has received independent research funding from Pfizer. FH has served on advisory boards or as speaker for Abbvie, Janssen, MSD, Takeda, Pfizer, Celltrion, Teva, Sandoz, Amgen and Pendopharm, and has received independent research funding from Janssen, Abbvie, Pfizer, and Takeda.
Figures

References
-
- Jess T, Rungoe C, Peyrin-Biroulet L.. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 2012;10:639–45. - PubMed
-
- Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B.. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013;19:789–99. - PubMed
-
- Olen O, Erichsen R, Sachs MC, et al.. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet 2020;395:123–31. - PubMed
-
- Olén O, Erichsen R, Sachs MC, et al.. Colorectal cancer in Crohn’s disease: a Scandinavian population-based cohort study. Clin Gastroenterol Hepatol 2020;5:475–84. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

