Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction
- PMID: 38741238
- PMCID: PMC11261224
- DOI: 10.3350/cmh.2024.0060
Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction
Erratum in
-
Erratum to 'Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction' [Clin Mol Hepatol 2024;30:539-560].Clin Mol Hepatol. 2024 Oct;30(4):1060-1065. doi: 10.3350/cmh.2024.0060e. Epub 2024 Jul 24. Clin Mol Hepatol. 2024. PMID: 39044466 Free PMC article. No abstract available.
Abstract
Background/aims: The major histocompatibility class II (MHC II) transactivator, known as CIITA, is induced by Interferon gamma (IFN-γ) and plays a well-established role in regulating the expression of class II MHC molecules in antigen-presenting cells.
Methods: Primary human hepatocytes (PHH) were isolated via therapeutic hepatectomy from two donors. The hepatocellular carcinoma (HCC) cell lines HepG2 and Huh7 were used for the mechanistic study, and HBV infection was performed in HepG2-NTCP cells. HBV DNA replication intermediates and secreted antigen levels were measured using Southern blotting and ELISA, respectively.
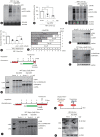
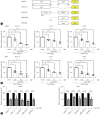
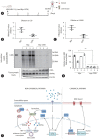
Results: We identified a non-canonical function of CIITA in the inhibition of hepatitis B virus (HBV) replication in both HCC cells and patient-derived PHH. Notably, in vivo experiments demonstrated that HBV DNA and secreted antigen levels were significantly decreased in mice injected with the CIITA construct. Mechanistically, CIITA inhibited HBV transcription and replication by suppressing the activity of HBV-specific enhancers/promoters. Indeed, CIITA exerts antiviral activity in hepatocytes through ERK1/2-mediated down-regulation of the expression of hepatocyte nuclear factor 1α (HNF1α) and HNF4α, which are essential factors for virus replication. In addition, silencing of CIITA significantly abolished the IFN-γ-mediated anti-HBV activity, suggesting that CIITA mediates the anti-HBV activity of IFN-γ to some extent. HBV X protein (HBx) counteracts the antiviral activity of CIITA via direct binding and impairing its function.
Conclusion: Our findings reveal a novel antiviral mechanism of CIITA that involves the modulation of the ERK pathway to restrict HBV transcription. Additionally, our results suggest the possibility of a new immune avoidance mechanism involving HBx.
Keywords: HBV X protein; Hepatitis B virus; Hepatocyte nuclear factor; Interferon-gamma; MHC class II transactivator.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures






Comment in
-
Class II transactivator restricts viral replication, extending its effect to HBV: Editorial on "Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction".Clin Mol Hepatol. 2024 Oct;30(4):724-727. doi: 10.3350/cmh.2024.0465. Epub 2024 Jul 3. Clin Mol Hepatol. 2024. PMID: 38957141 Free PMC article. No abstract available.
References
-
- Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182:104925. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

