Novel Alaninamide Derivatives with Drug-like Potential for Development as Antiseizure and Antinociceptive Therapies─In Vitro and In Vivo Characterization
- PMID: 38741575
- PMCID: PMC11157491
- DOI: 10.1021/acschemneuro.4c00013
Novel Alaninamide Derivatives with Drug-like Potential for Development as Antiseizure and Antinociceptive Therapies─In Vitro and In Vivo Characterization
Abstract
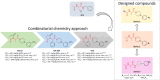
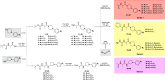
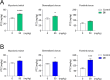
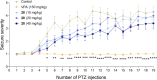
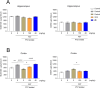
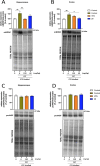
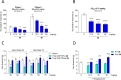
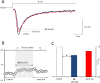
In the present study, a series of original alaninamide derivatives have been designed applying a combinatorial chemistry approach, synthesized, and characterized in the in vivo and in vitro assays. The obtained molecules showed potent and broad-spectrum activity in basic seizure models, namely, the maximal electroshock (MES) test, the 6 Hz (32 mA) seizure model, and notably, the 6 Hz (44 mA) model of pharmacoresistant seizures. Most potent compounds 26 and 28 displayed the following pharmacological values: ED50 = 64.3 mg/kg (MES), ED50 = 15.6 mg/kg (6 Hz, 32 mA), ED50 = 29.9 mg/kg (6 Hz, 44 mA), and ED50 = 34.9 mg/kg (MES), ED50 = 12.1 mg/kg (6 Hz, 32 mA), ED50 = 29.5 mg/kg (6 Hz, 44 mA), respectively. Additionally, 26 and 28 were effective in the ivPTZ seizure threshold test and had no influence on the grip strength. Moreover, lead compound 28 was tested in the PTZ-induced kindling model, and then, its influence on glutamate and GABA levels in the hippocampus and cortex was evaluated by the high-performance liquid chromatography (HPLC) method. In addition, 28 revealed potent efficacy in formalin-induced tonic pain, capsaicin-induced pain, and oxaliplatin- and streptozotocin-induced peripheral neuropathy. Pharmacokinetic studies and in vitro ADME-Tox data proved favorable drug-like properties of 28. The patch-clamp recordings in rat cortical neurons showed that 28 at a concentration of 10 μM significantly inhibited fast sodium currents. Therefore, 28 seems to be an interesting candidate for future preclinical development in epilepsy and pain indications.
Keywords: ADME-Tox properties; antinociceptive activity; antiseizure activity; epilepsy; hybrid molecules; neuropathic pain.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Discovery and Profiling of New Multimodal Phenylglycinamide Derivatives as Potent Antiseizure and Antinociceptive Drug Candidates.ACS Chem Neurosci. 2024 Sep 4;15(17):3228-3256. doi: 10.1021/acschemneuro.4c00438. Epub 2024 Aug 21. ACS Chem Neurosci. 2024. PMID: 39166702 Free PMC article.
-
New Phenylglycinamide Derivatives with Hybrid Structure as Candidates for New Broad-Spectrum Anticonvulsants.Cells. 2022 Jun 7;11(12):1862. doi: 10.3390/cells11121862. Cells. 2022. PMID: 35740990 Free PMC article.
-
Identification of New Compounds with Anticonvulsant and Antinociceptive Properties in a Group of 3-substituted (2,5-dioxo-pyrrolidin-1-yl)(phenyl)-Acetamides.Int J Mol Sci. 2021 Dec 3;22(23):13092. doi: 10.3390/ijms222313092. Int J Mol Sci. 2021. PMID: 34884898 Free PMC article.
-
Novel Hybrid Anticonvulsants Derived from Pyrrolidine-2,5-dione Scaffold with Broad Spectrum of Activity in the Preclinical Studies.Curr Top Med Chem. 2017;17(8):858-874. doi: 10.2174/1568026616666160927153025. Curr Top Med Chem. 2017. PMID: 27697054 Review.
-
The Search for New Screening Models of Pharmacoresistant Epilepsy: Is Induction of Acute Seizures in Epileptic Rodents a Suitable Approach?Neurochem Res. 2017 Jul;42(7):1926-1938. doi: 10.1007/s11064-016-2025-7. Epub 2016 Aug 8. Neurochem Res. 2017. PMID: 27502939 Review.
Cited by
-
Development of Novel Alaninamide Derivatives with Anticonvulsant Activity and Favorable Safety Profiles in Animal Models.Int J Mol Sci. 2024 Sep 12;25(18):9861. doi: 10.3390/ijms25189861. Int J Mol Sci. 2024. PMID: 39337345 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

