Transcriptomic Insights into Different Stimulation Intensity of Electroacupuncture in Treating COPD in Rat Models
- PMID: 38741612
- PMCID: PMC11090121
- DOI: 10.2147/JIR.S458580
Transcriptomic Insights into Different Stimulation Intensity of Electroacupuncture in Treating COPD in Rat Models
Abstract
Background: Electroacupuncture (EA), with varying stimulation intensities, has demonstrated therapeutic potentials in both animal and clinical studies for the treatment of chronic obstructive pulmonary disease (COPD). However, a comprehensive investigation of the intensity-related effects, particularly 1mA and 3mA of EA, and the underlying mechanisms remains lacking.
Methods: A COPD rat model was established by prolonged exposure to cigarette smoke and intermittent intratracheal instillation of lipopolysaccharide. EA treatment was administered at acupoints BL13 (Feishu) and ST36 (Zusanli), 20 minutes daily for 2 weeks, with intensities of 1mA and 3mA. EA effectiveness was evaluated by pulmonary function, histopathological change, serum level of inflammatory cytokines, and level of oxidative stress markers in serum and lung tissues. Transcriptome profiling and weighted gene co-expression network analysis (WGCNA) were performed to reveal gene expression patterns and identify hub genes. Real-time quantitative PCR (RT-qPCR) and Western blot (WB) were performed to detect the mRNA and protein expression levels, respectively.
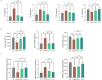
Results: EA at both 1mA and 3mA exerted differing therapeutic effects by improving lung function and reducing inflammation and oxidative stress in COPD rats. Transcriptome analysis revealed distinct expression patterns between the two groups, functionally corresponding to shared and intensity-specific (1mA and 3mA) enriched pathways. Eight candidate genes were identified, including Aqp9, Trem1, Mrc1, and Gpnmb that were downregulated by EA and upregulated in COPD. Notably, Msr1 and Slc26a4 exclusively downregulated in EA-1mA, while Pde3a and Bmp6 upregulated solely in EA-3mA. WGCNA constructed 5 key modules and elucidated the module-trait relationship, with the aforementioned 8 genes being highlighted. Additionally, their mRNA and protein levels were validated by RT-qPCR and WB.
Conclusion: Our results demonstrated that 1mA and 3mA intensities induce distinct gene expression patterns at the transcriptional level, associated with shared and 1mA vs 3mA-specific enriched pathways. Genes Mrc1, Gpnmb, Trem1, and Aqp9 emerge as promising targets, and further studies are needed to elucidate their functional consequences in COPD.
Keywords: chronic obstructive pulmonary disease; electroacupuncture; intensity; transcriptome profiling; weighted gene co-expression network analysis.
© 2024 Liu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

