Catalytic asymmetric carbenoid α-C-H insertion of ether
- PMID: 38741618
- PMCID: PMC11090019
- DOI: 10.1039/d4ra02206h
Catalytic asymmetric carbenoid α-C-H insertion of ether
Abstract
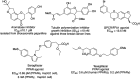
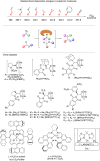
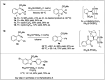
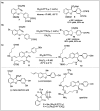
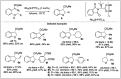
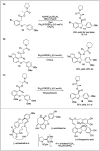
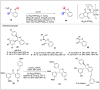
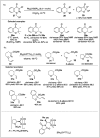
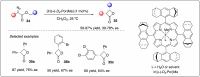
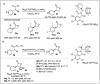
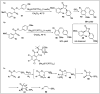
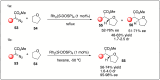
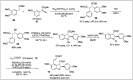
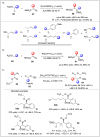
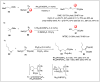
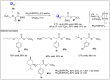
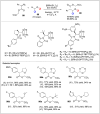
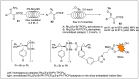
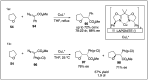
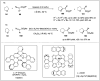
Significant advancements have been made in catalytic asymmetric α-C-H bond functionalization of ethers via carbenoid insertion over the past decade. Effective asymmetric catalytic systems, featuring a range of chiral metal catalysts, have been established for the enantioselective synthesis of diverse ether substrates. This has led to the generation of various enantioenriched, highly functionalized oxygen-containing structural motifs, facilitating their application in the asymmetric synthesis of bioactive natural products.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Synthesis of Planar Chiral Ferrocenes via Transition-Metal-Catalyzed Direct C-H Bond Functionalization.Acc Chem Res. 2017 Feb 21;50(2):351-365. doi: 10.1021/acs.accounts.6b00573. Epub 2017 Jan 25. Acc Chem Res. 2017. PMID: 28121428
-
Transition-metal-catalyzed enantioselective heteroatom-hydrogen bond insertion reactions.Acc Chem Res. 2012 Aug 21;45(8):1365-77. doi: 10.1021/ar300051u. Epub 2012 May 31. Acc Chem Res. 2012. PMID: 22651217
-
Chiral Aldehyde Catalysis-Enabled Asymmetric α-Functionalization of Activated Primary Amines.Acc Chem Res. 2024 Mar 5;57(5):776-794. doi: 10.1021/acs.accounts.3c00804. Epub 2024 Feb 21. Acc Chem Res. 2024. PMID: 38381559
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
-
Catalytic Enantioselective Dihalogenation in Total Synthesis.Acc Chem Res. 2018 May 15;51(5):1260-1271. doi: 10.1021/acs.accounts.8b00064. Epub 2018 Apr 17. Acc Chem Res. 2018. PMID: 29664281 Free PMC article. Review.
References
-
- Docherty J. H. Lister T. M. Mcarthur G. Findlay M. T. Domingo-Legarda P. Kenyon J. Choudhary S. Larrosa I. Transition-metal-catalyzed C–H bond activation for the formation of C–C bonds in complex molecules. Chem. Rev. 2023;123:7692–7760. - PMC - PubMed
- Lapuh M. I. Mazeh S. Besset T. Chiral transient directing groups in transition-metal-catalyzed enantioselective C–H bond functionalization. ACS Catal. 2020;10:12898–12919.
-
- Santiago J. V. Machado A. H. L. Enantioselective carbenoid insertion into C(sp3)–H bonds. Beilstein J. Org. Chem. 2016;12:882–902. - PMC - PubMed
- Gandhi S. Catalytic enantioselective cross dehydrogenative coupling of sp3 C–H of heterocycles. Org. Biomol. Chem. 2019;17:9683. - PubMed
- Zhang S.-Y. Zhang F.-M. Tu Y.-Q. Direct Sp3 α-C–H activation and functionalization of alcohol and ether. Chem. Soc. Rev. 2011;40:1937–1949. - PubMed
-
- Silberrad O. Roy C. S. XXIV.—Gradual decomposition of ethyl diazoacetate. J. Chem. Soc., Trans. 1906;89:179–182.
-
- Yates P. The copper-catalyzed decomposition of diazoketones. J. Am. Chem. Soc. 1952;74:5376–5381.
- Scott L. T. DeCicco G. J. Intermolecular carbon-hydrogen insertion of copper carbenoids. J. Am. Chem. Soc. 1974;96:322–323.
-
- Doyle M. P. Duffy R. Ratnikov M. Zhou L. Catalytic carbene insertion into C–H bonds. Chem. Rev. 2010;110:704–724. - PubMed
- Bergstrom B. D. Nickerson L. A. Shaw J. T. Souza L. W. Transition metal catalyzed insertion reactions with donor/donor carbenes. Angew. Chem., Int. Ed. 2021;60:6864–6878. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

