Mitochondrial F0F1-ATP synthase governs the induction of mitochondrial fission
- PMID: 38741710
- PMCID: PMC11089353
- DOI: 10.1016/j.isci.2024.109808
Mitochondrial F0F1-ATP synthase governs the induction of mitochondrial fission
Abstract
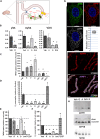
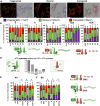
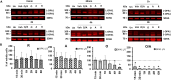
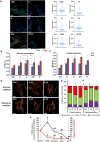
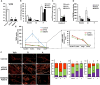
Mitochondrial dynamics is a process that balances fusion and fission events, the latter providing a mechanism for segregating dysfunctional mitochondria. Fission is controlled by the mitochondrial membrane potential (ΔΨm), optic atrophy 1 (OPA1) cleavage, and DRP1 recruitment. It is thought that this process is closely linked to the activity of the mitochondrial respiratory chain (MRC). However, we report here that MRC inhibition does not decrease ΔΨm nor increase fission, as evidenced by hyperconnected mitochondria. Conversely, blocking F0F1-ATP synthase activity induces fragmentation. We show that the F0F1-ATP synthase is sensing the inhibition of MRC activity by immediately promoting its reverse mode of action to hydrolyze matrix ATP and restoring ΔΨm, thus preventing fission. While this reverse mode is expected to be inhibited by the ATPase inhibitor ATPIF1, we show that this sensing is independent of this factor. We have unraveled an unexpected role of F0F1-ATP synthase in controlling the induction of fission by sensing and maintaining ΔΨm.
Keywords: Biochemistry; Cell biology; Functional aspects of cell biology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Rojo M., Legros F., Chateau D., Lombès A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 2002;115:1663–1674. - PubMed
-
- Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000;26:207–210. doi: 10.1038/79936. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

