Lipid metabolism disorder in diabetic kidney disease
- PMID: 38742197
- PMCID: PMC11089115
- DOI: 10.3389/fendo.2024.1336402
Lipid metabolism disorder in diabetic kidney disease
Abstract
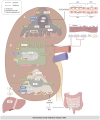
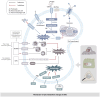
Diabetic kidney disease (DKD), a significant complication associated with diabetes mellitus, presents limited treatment options. The progression of DKD is marked by substantial lipid disturbances, including alterations in triglycerides, cholesterol, sphingolipids, phospholipids, lipid droplets, and bile acids (BAs). Altered lipid metabolism serves as a crucial pathogenic mechanism in DKD, potentially intertwined with cellular ferroptosis, lipophagy, lipid metabolism reprogramming, and immune modulation of gut microbiota (thus impacting the liver-kidney axis). The elucidation of these mechanisms opens new potential therapeutic pathways for DKD management. This research explores the link between lipid metabolism disruptions and DKD onset.
Keywords: diabetic kidney disease; ferroptosis; gut microbiota; lipid metabolism; metabolic reprogramming; treatment.
Copyright © 2024 Han, Du, Zhu, Wang, Zheng and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

