Diacylglycerol kinase is a keystone regulator of signaling relevant to the pathophysiology of asthma
- PMID: 38742284
- PMCID: PMC11380957
- DOI: 10.1152/ajplung.00091.2024
Diacylglycerol kinase is a keystone regulator of signaling relevant to the pathophysiology of asthma
Abstract
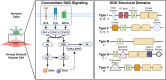
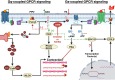
Signal transduction by G protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs) and immunoreceptors converge at the activation of phospholipase C (PLC) for the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). This is a point for second-messenger bifurcation where DAG via protein kinase C (PKC) and IP3 via calcium activate distinct protein targets and regulate cellular functions. IP3 signaling is regulated by multiple calcium influx and efflux proteins involved in calcium homeostasis. A family of lipid kinases belonging to DAG kinases (DGKs) converts DAG to phosphatidic acid (PA), negatively regulating DAG signaling and pathophysiological functions. PA, through a series of biochemical reactions, is recycled to produce new molecules of PIP2. Therefore, DGKs act as a central switch in terminating DAG signaling and resynthesis of membrane phospholipids precursor. Interestingly, calcium and PKC regulate the activation of α and ζ isoforms of DGK that are predominantly expressed in airway and immune cells. Thus, DGK forms a feedback and feedforward control point and plays a crucial role in fine-tuning phospholipid stoichiometry, signaling, and functions. In this review, we discuss the previously underappreciated complex and intriguing DAG/DGK-driven mechanisms in regulating cellular functions associated with asthma, such as contraction and proliferation of airway smooth muscle (ASM) cells and inflammatory activation of immune cells. We highlight the benefits of manipulating DGK activity in mitigating salient features of asthma pathophysiology and shed light on DGK as a molecule of interest for heterogeneous diseases such as asthma.
Keywords: DAG; airway smooth muscle; asthma; diacylglycerol kinase; remodeling.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste JC, Lemanske RF Jr, Levy ML, O'Byrne PM, Paggiaro P, Pedersen SE, Pizzichini E, Soto-Quiroz M, Szefler SJ, Wong GW, FitzGerald JM. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 46: 622–639, 2015. doi: 10.1183/13993003.00853-2015. - DOI - PMC - PubMed
-
- Cohen SG. Sir John Floyer (1649–1734) British physician and pioneer clinical investigator. Allergy Proc 16: 328–329, 1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

