Skeletal muscle hypertrophy rewires glucose metabolism: An experimental investigation and systematic review
- PMID: 38742477
- PMCID: PMC11154753
- DOI: 10.1002/jcsm.13468
Skeletal muscle hypertrophy rewires glucose metabolism: An experimental investigation and systematic review
Abstract
Background: Proliferating cancer cells shift their metabolism towards glycolysis, even in the presence of oxygen, to especially generate glycolytic intermediates as substrates for anabolic reactions. We hypothesize that a similar metabolic remodelling occurs during skeletal muscle hypertrophy.
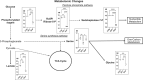
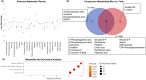
Methods: We used mass spectrometry in hypertrophying C2C12 myotubes in vitro and plantaris mouse muscle in vivo and assessed metabolomic changes and the incorporation of the [U-13C6]glucose tracer. We performed enzyme inhibition of the key serine synthesis pathway enzyme phosphoglycerate dehydrogenase (Phgdh) for further mechanistic analysis and conducted a systematic review to align any changes in metabolomics during muscle growth with published findings. Finally, the UK Biobank was used to link the findings to population level.
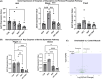
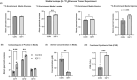
Results: The metabolomics analysis in myotubes revealed insulin-like growth factor-1 (IGF-1)-induced altered metabolite concentrations in anabolic pathways such as pentose phosphate (ribose-5-phosphate/ribulose-5-phosphate: +40%; P = 0.01) and serine synthesis pathway (serine: -36.8%; P = 0.009). Like the hypertrophy stimulation with IGF-1 in myotubes in vitro, the concentration of the dipeptide l-carnosine was decreased by 26.6% (P = 0.001) during skeletal muscle growth in vivo. However, phosphorylated sugar (glucose-6-phosphate, fructose-6-phosphate or glucose-1-phosphate) decreased by 32.2% (P = 0.004) in the overloaded muscle in vivo while increasing in the IGF-1-stimulated myotubes in vitro. The systematic review revealed that 10 metabolites linked to muscle hypertrophy were directly associated with glycolysis and its interconnected anabolic pathways. We demonstrated that labelled carbon from [U-13C6]glucose is increasingly incorporated by ~13% (P = 0.001) into the non-essential amino acids in hypertrophying myotubes, which is accompanied by an increased depletion of media serine (P = 0.006). The inhibition of Phgdh suppressed muscle protein synthesis in growing myotubes by 58.1% (P < 0.001), highlighting the importance of the serine synthesis pathway for maintaining muscle size. Utilizing data from the UK Biobank (n = 450 243), we then discerned genetic variations linked to the serine synthesis pathway (PHGDH and PSPH) and to its downstream enzyme (SHMT1), revealing their association with appendicular lean mass in humans (P < 5.0e-8).
Conclusions: Understanding the mechanisms that regulate skeletal muscle mass will help in developing effective treatments for muscle weakness. Our results provide evidence for the metabolic rewiring of glycolytic intermediates into anabolic pathways during muscle growth, such as in serine synthesis.
Keywords: Warburg effect; lactate; metabolomics; resistance exercise; serine synthesis pathway.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Wackerhage H, Schoenfeld BJ, Hamilton DL, Lehti M, Hulmi JJ. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J Appl Physiol 2019;126:30–43. - PubMed
-
- Goodman CA. Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J Appl Physiol 2019;127:581–590. - PubMed
-
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27:441–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

