Fluorescent small molecule donors
- PMID: 38742651
- PMCID: PMC11181996
- DOI: 10.1039/d3cs00124e
Fluorescent small molecule donors
Abstract
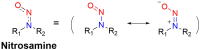
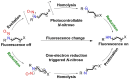
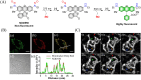
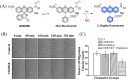
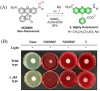
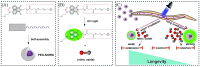
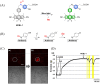
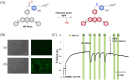
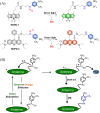
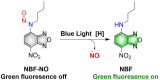
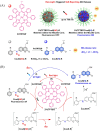
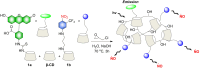
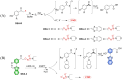
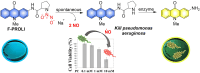
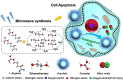
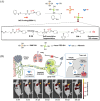
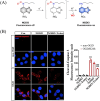
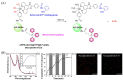
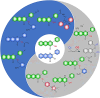
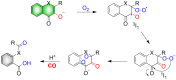
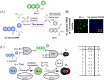
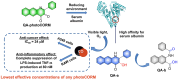
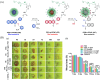
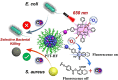
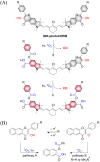
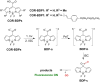
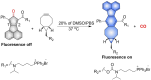
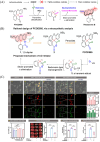
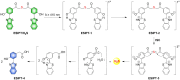
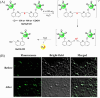
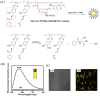
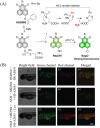
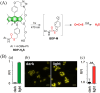
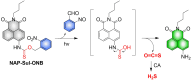
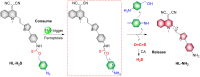
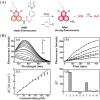
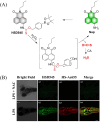
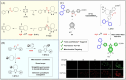
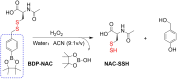
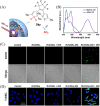
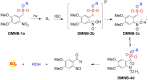
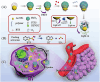
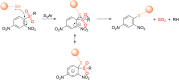
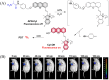
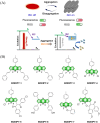
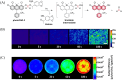
Small molecule donors (SMDs) play subtle roles in the signaling mechanism and disease treatments. While many excellent SMDs have been developed, dosage control, targeted delivery, spatiotemporal feedback, as well as the efficiency evaluation of small molecules are still key challenges. Accordingly, fluorescent small molecule donors (FSMDs) have emerged to meet these challenges. FSMDs enable controllable release and non-invasive real-time monitoring, providing significant advantages for drug development and clinical diagnosis. Integration of FSMDs with chemotherapeutic, photodynamic or photothermal properties can take full advantage of each mode to enhance therapeutic efficacy. Given the remarkable properties and the thriving development of FSMDs, we believe a review is needed to summarize the design, triggering strategies and tracking mechanisms of FSMDs. With this review, we compiled FSMDs for most small molecules (nitric oxide, carbon monoxide, hydrogen sulfide, sulfur dioxide, reactive oxygen species and formaldehyde), and discuss recent progress concerning their molecular design, structural classification, mechanisms of generation, triggered release, structure-activity relationships, and the fluorescence response mechanism. Firstly, from the large number of fluorescent small molecular donors available, we have organized the common structures for producing different types of small molecules, providing a general strategy for the development of FSMDs. Secondly, we have classified FSMDs in terms of the respective donor types and fluorophore structures. Thirdly, we discuss the mechanisms and factors associated with the controlled release of small molecules and the regulation of the fluorescence responses, from which universal guidelines for optical properties and structure rearrangement were established, mainly involving light-controlled, enzyme-activated, reactive oxygen species-triggered, biothiol-triggered, single-electron reduction, click chemistry, and other triggering mechanisms. Fourthly, representative applications of FSMDs for trackable release, and evaluation monitoring, as well as for visible in vivo treatment are outlined, to illustrate the potential of FSMDs in drug screening and precision medicine. Finally, we discuss the opportunities and remaining challenges for the development of FSMDs for practical and clinical applications, which we anticipate will stimulate the attention of researchers in the diverse fields of chemistry, pharmacology, chemical biology and clinical chemistry. With this review, we hope to impart new understanding thereby enabling the rapid development of the next generation of FSMDs.
Conflict of interest statement
There are no conflicts to declare.
Figures









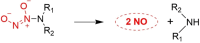









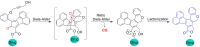





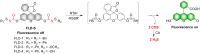
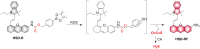





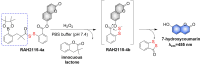













References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

