Multistate Method to Efficiently Account for Tautomerism and Protonation in Alchemical Free-Energy Calculations
- PMID: 38742760
- PMCID: PMC11137823
- DOI: 10.1021/acs.jctc.4c00370
Multistate Method to Efficiently Account for Tautomerism and Protonation in Alchemical Free-Energy Calculations
Abstract
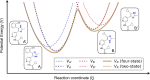
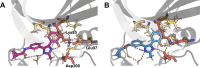
The majority of drug-like molecules contain at least one ionizable group, and many common drug scaffolds are subject to tautomeric equilibria. Thus, these compounds are found in a mixture of protonation and/or tautomeric states at physiological pH. Intrinsically, standard classical molecular dynamics (MD) simulations cannot describe such equilibria between states, which negatively impacts the prediction of key molecular properties in silico. Following the formalism described by de Oliveira and co-workers (J. Chem. Theory Comput. 2019, 15, 424-435) to consider the influence of all states on the binding process based on alchemical free-energy calculations, we demonstrate in this work that the multistate method replica-exchange enveloping distribution sampling (RE-EDS) is well suited to describe molecules with multiple protonation and/or tautomeric states in a single simulation. We apply our methodology to a series of eight inhibitors of factor Xa with two protonation states and a series of eight inhibitors of glycogen synthase kinase 3β (GSK3β) with two tautomeric states. In particular, we show that given a sufficient phase-space overlap between the states, RE-EDS is computationally more efficient than standard pairwise free-energy methods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

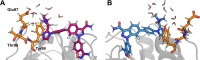



Similar articles
-
Rigorous Free Energy Perturbation Approach to Estimating Relative Binding Affinities between Ligands with Multiple Protonation and Tautomeric States.J Chem Theory Comput. 2019 Jan 8;15(1):424-435. doi: 10.1021/acs.jctc.8b00826. Epub 2018 Dec 26. J Chem Theory Comput. 2019. PMID: 30537823
-
Accelerating Alchemical Free Energy Prediction Using a Multistate Method: Application to Multiple Kinases.J Chem Inf Model. 2023 Nov 27;63(22):7133-7147. doi: 10.1021/acs.jcim.3c01469. Epub 2023 Nov 10. J Chem Inf Model. 2023. PMID: 37948537 Free PMC article.
-
Replica exchange enveloping distribution sampling (RE-EDS): A robust method to estimate multiple free-energy differences from a single simulation.J Chem Phys. 2016 Oct 21;145(15):154114. doi: 10.1063/1.4964781. J Chem Phys. 2016. PMID: 27782485
-
Relative free-energy calculations for scaffold hopping-type transformations with an automated RE-EDS sampling procedure.J Comput Aided Mol Des. 2022 Feb;36(2):117-130. doi: 10.1007/s10822-021-00436-z. Epub 2022 Jan 3. J Comput Aided Mol Des. 2022. PMID: 34978000 Free PMC article.
-
Protonation States in molecular dynamics simulations of peptide folding and binding.Curr Pharm Des. 2013;19(23):4173-81. doi: 10.2174/1381612811319230003. Curr Pharm Des. 2013. PMID: 23170889 Review.
Cited by
-
Efficient Multistate Free-Energy Calculations with QM/MM Accuracy Using Replica-Exchange Enveloping Distribution Sampling.J Phys Chem B. 2025 Jun 19;129(24):5948-5960. doi: 10.1021/acs.jpcb.5c02086. Epub 2025 Jun 6. J Phys Chem B. 2025. PMID: 40479643 Free PMC article.
-
Fast and Accurate Prediction of Tautomer Ratios in Aqueous Solution via a Siamese Neural Network.J Chem Theory Comput. 2025 Mar 25;21(6):3132-3141. doi: 10.1021/acs.jctc.5c00041. Epub 2025 Mar 16. J Chem Theory Comput. 2025. PMID: 40091187 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
