Synergistic effects of sulopenem in combination with cefuroxime or durlobactam against Mycobacterium abscessus
- PMID: 38742824
- PMCID: PMC11237399
- DOI: 10.1128/mbio.00609-24
Synergistic effects of sulopenem in combination with cefuroxime or durlobactam against Mycobacterium abscessus
Abstract
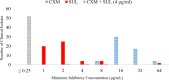
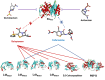
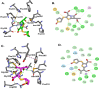
Mycobacterium abscessus (Mab) affects patients with immunosuppression or underlying structural lung diseases such as cystic fibrosis (CF). Additionally, Mab poses clinical challenges due to its resistance to multiple antibiotics. Herein, we investigated the synergistic effect of dual β-lactams [sulopenem and cefuroxime (CXM)] or the combination of sulopenem and CXM with β-lactamase inhibitors [BLIs-avibactam (AVI) or durlobactam (DUR)]. The sulopenem-CXM combination yielded low minimum inhibitory concentration (MIC) values for 54 clinical Mab isolates and ATCC19977 (MIC50 and MIC90 ≤0.25 µg/mL). Similar synergistic effects were observed in time-kill studies conducted at concentrations achievable in clinical settings. Sulopenem-CXM outperformed monotherapy, yielding ~1.5 Log10 CFU/mL reduction during 10 days. Addition of BLIs enhanced this antibacterial effect, resulting in an additional reduction of CFUs (~3 Log10 for sulopenem-CXM and AVI and ~4 Log10 for sulopenem-DUR). Exploration of the potential mechanisms of the synergy focused on their interactions with L,D-transpeptidases (Ldts; LdtMab1-LdtMab4), penicillin-binding-protein B (PBP B), and D,D-carboxypeptidase (DDC). Acyl complexes, identified via mass spectrometry analysis, demonstrated the binding of sulopenem with LdtMab2-LdtMab4, DDC, and PBP B and CXM with LdtMab2 and PBP B. Molecular docking and mass spectrometry data suggest the formation of a covalent adduct between sulopenem and LdtMab2 after the nucleophilic attack of the cysteine residue at the β-lactam carbonyl carbon, leading to the cleavage of the β-lactam ring and the establishment of a thioester bond linking the LdtMab2 with sulopenem. In conclusion, we demonstrated the biochemical basis of the synergy of sulopenem-CXM with or without BLIs. These findings potentially broaden the selection of oral therapeutic agents to combat Mab.
Importance: Treating infections from Mycobacterium abscessus (Mab), particularly those resistant to common antibiotics like macrolides, is notoriously difficult, akin to a never-ending struggle for healthcare providers. The rate of treatment failure is even higher than that seen with multidrug-resistant tuberculosis. The role of combination β-lactams in inhibiting L,D-transpeptidation, the major peptidoglycan crosslink reaction in Mab, is an area of intense investigation, and clinicians have utilized this approach in the treatment of macrolide-resistant Mab, with reports showing clinical success. In our study, we found that cefuroxime and sulopenem, when used together, display a significant synergistic effect. If this promising result seen in lab settings, translates well into real-world clinical effectiveness, it could revolutionize current treatment methods. This combination could either replace the need for more complex intravenous medications or serve as a "step down" to an oral medication regimen. Such a shift would be much easier for patients to manage, enhancing their comfort and likelihood of sticking to the treatment plan, which could lead to better outcomes in tackling these tough infections. Our research delved into how these drugs inhibit cell wall synthesis, examined time-kill data and binding studies, and provided a scientific basis for the observed synergy in cell-based assays.
Keywords: Mycobacterium abscessus; dual β-lactams; oral carbapenem; sulopenem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
