Effect of Cangrelor on Infarct Size in ST-Segment-Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention: A Randomized Controlled Trial (The PITRI Trial)
- PMID: 38742915
- PMCID: PMC11224569
- DOI: 10.1161/CIRCULATIONAHA.124.068938
Effect of Cangrelor on Infarct Size in ST-Segment-Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention: A Randomized Controlled Trial (The PITRI Trial)
Abstract
Background: The administration of intravenous cangrelor at reperfusion achieves faster onset of platelet P2Y12 inhibition than oral ticagrelor and has been shown to reduce myocardial infarction (MI) size in the preclinical setting. We hypothesized that the administration of cangrelor at reperfusion will reduce MI size and prevent microvascular obstruction in patients with ST-segment-elevation MI undergoing primary percutaneous coronary intervention.
Methods: This was a phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial conducted between November 2017 to November 2021 in 6 cardiac centers in Singapore. Patients were randomized to receive either cangrelor or placebo initiated before the primary percutaneous coronary intervention procedure on top of oral ticagrelor. The key exclusion criteria included presenting <6 hours of symptom onset; previous MI and stroke or transient ischemic attack; on concomitant oral anticoagulants; and a contraindication for cardiovascular magnetic resonance. The primary efficacy end point was acute MI size by cardiovascular magnetic resonance within the first week expressed as percentage of the left ventricle mass (%LVmass). Microvascular obstruction was identified as areas of dark core of hypoenhancement within areas of late gadolinium enhancement. The primary safety end point was Bleeding Academic Research Consortium-defined major bleeding in the first 48 hours. Continuous variables were compared by Mann-Whitney U test (reported as median [first quartile-third quartile]), and categorical variables were compared by Fisher exact test. A 2-sided P<0.05 was considered statistically significant.
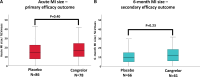
Results: Of 209 recruited patients, 164 patients (78%) completed the acute cardiovascular magnetic resonance scan. There were no significant differences in acute MI size (placebo, 14.9% [7.3-22.6] %LVmass versus cangrelor, 16.3 [9.9-24.4] %LVmass; P=0.40) or the incidence (placebo, 48% versus cangrelor, 47%; P=0.99) and extent of microvascular obstruction (placebo, 1.63 [0.60-4.65] %LVmass versus cangrelor, 1.18 [0.53-3.37] %LVmass; P=0.46) between placebo and cangrelor despite a 2-fold decrease in platelet reactivity with cangrelor. There were no Bleeding Academic Research Consortium-defined major bleeding events in either group in the first 48 hours.
Conclusions: Cangrelor administered at the time of primary percutaneous coronary intervention did not reduce acute MI size or prevent microvascular obstruction in patients with ST-segment-elevation MI given oral ticagrelor despite a significant reduction of platelet reactivity during the percutaneous coronary intervention procedure.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03102723.
Keywords: ST-segment–elevation myocardial infarction; cangrelor; cardiovascular magnetic resonance; microvascular obstruction; myocardial infarct size; primary percutaneous coronary intervention; ticagrelor.
Conflict of interest statement
L.T. is on the Astra Zeneca international advisory board of management of adverse events with the new antibody drug conjugate T-DXd in Asian patients with metastatic breast cancer, Roche Singapore immunotherapy in early stage NSCLC patient journey advisory board. L.T. has received a Philips speaker honorarium in kind and a Siemens Healthineers speaker honorarium. Y.K.K. has received research funding from Amgen, Astra Zeneca, Abbott Vascular, Bayer, Boston Scientific, Shockwave Medical, and Novartis (via institution); consulting fees from Abbott Vascular, Medtronic, Novartis, and Peijia Medical; and speaker fees from Shockwave Medical, Abbott Vascular, Boston Scientific, Medtronic, Alvimedica, Biotronik, Orbus Neich, Amgen, Novartis, Astra Zeneca, Microport, Terumo, and Omnicare. Y.K.K. is also cofounder and owns equity in Trisail, for which OrbusNeich is an investor. D.J.H. has received consultant fees from Faraday Pharmaceuticals Inc and Boehringer Ingelheim International GmbH, honoraria from Servier, and research funding from Astra Zeneca and Merck Sharp & Dohme Corp. C.Y.C. has received speaker fees from Novartis and consultancy fees from Boston Scientific and Philips. The other authors report no conflicts.
Figures


References
-
- Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489 - PubMed
-
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667 - PubMed
-
- Krug A, du Mesnil de Rochemont W, Korb G. Blood supply of the myocardium after temporary coronary occlusion. Circ Res. 1966;19:57–62. - PubMed
-
- White SK, Sado DM, Flett AS, Moon JC. Characterising the myocardial interstitial space: the clinical relevance of non-invasive imaging. Heart. 2012;98:773–779. doi: 10.1136/heartjnl-2011-301515 - PubMed
-
- Bulluck H, Hausenloy DJ. Microvascular obstruction: the bane of myocardial reperfusion. Rev Esp Cardiol (Engl Ed). 2015;68:919–920. doi: 10.1016/j.rec.2015.06.022 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

