RFX6 haploinsufficiency predisposes to diabetes through impaired beta cell function
- PMID: 38743124
- PMCID: PMC11343796
- DOI: 10.1007/s00125-024-06163-y
RFX6 haploinsufficiency predisposes to diabetes through impaired beta cell function
Abstract
Aims/hypothesis: Regulatory factor X 6 (RFX6) is crucial for pancreatic endocrine development and differentiation. The RFX6 variant p.His293LeufsTer7 is significantly enriched in the Finnish population, with almost 1:250 individuals as a carrier. Importantly, the FinnGen study indicates a high predisposition for heterozygous carriers to develop type 2 and gestational diabetes. However, the precise mechanism of this predisposition remains unknown.
Methods: To understand the role of this variant in beta cell development and function, we used CRISPR technology to generate allelic series of pluripotent stem cells. We created two isogenic stem cell models: a human embryonic stem cell model; and a patient-derived stem cell model. Both were differentiated into pancreatic islet lineages (stem-cell-derived islets, SC-islets), followed by implantation in immunocompromised NOD-SCID-Gamma mice.
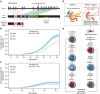
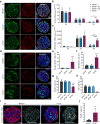
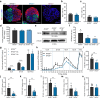
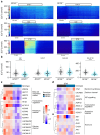
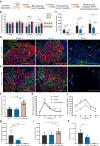
Results: Stem cell models of the homozygous variant RFX6-/- predictably failed to generate insulin-secreting pancreatic beta cells, mirroring the phenotype observed in Mitchell-Riley syndrome. Notably, at the pancreatic endocrine stage, there was an upregulation of precursor markers NEUROG3 and SOX9, accompanied by increased apoptosis. Intriguingly, heterozygous RFX6+/- SC-islets exhibited RFX6 haploinsufficiency (54.2% reduction in protein expression), associated with reduced beta cell maturation markers, altered calcium signalling and impaired insulin secretion (62% and 54% reduction in basal and high glucose conditions, respectively). However, RFX6 haploinsufficiency did not have an impact on beta cell number or insulin content. The reduced insulin secretion persisted after in vivo implantation in mice, aligning with the increased risk of variant carriers to develop diabetes.
Conclusions/interpretation: Our allelic series isogenic SC-islet models represent a powerful tool to elucidate specific aetiologies of diabetes in humans, enabling the sensitive detection of aberrations in both beta cell development and function. We highlight the critical role of RFX6 in augmenting and maintaining the pancreatic progenitor pool, with an endocrine roadblock and increased cell death upon its loss. We demonstrate that RFX6 haploinsufficiency does not affect beta cell number or insulin content but does impair function, predisposing heterozygous carriers of loss-of-function variants to diabetes.
Data availability: Ultra-deep bulk RNA-seq data for pancreatic differentiation stages 3, 5 and 7 of H1 RFX6 genotypes are deposited in the Gene Expression Omnibus database with accession code GSE234289. Original western blot images are deposited at Mendeley ( https://data.mendeley.com/datasets/g75drr3mgw/2 ).
Keywords: Beta cells; Glucose-stimulated insulin secretion; Isogenic allelic series models; Monogenic diabetes; Stem-cell-derived islets; Type 2 diabetes.
© 2024. The Author(s).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

