Changes in Basal and Bolus Insulin Requirements with Tirzepatide as an Adjunctive Therapy in Adults with Type 1 Diabetes Using Tandem Control-IQ
- PMID: 38743306
- PMCID: PMC11211304
- DOI: 10.1007/s13300-024-01592-9
Changes in Basal and Bolus Insulin Requirements with Tirzepatide as an Adjunctive Therapy in Adults with Type 1 Diabetes Using Tandem Control-IQ
Abstract
Introduction: This study was aimed at investigating changes in insulin requirements and glycemic outcomes in adults with type 1 diabetes (T1D) using Control IQ (Tandem Diabetes) automated insulin delivery system (AID) over 8 months of tirzepatide treatment.
Methods: In this single-center, observational study, we collected demographic, A1c, weight, sensor glucose, and insulin dose data for adults with T1D who were using AID and initiated tirzepatide adjunct therapy for clinical indications (n = 11, median age 37, 64% female and mean body mass index of 39.6 kg/m2). Data were compared from baseline and over 8 months.
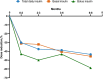
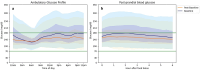
Results: Within 2 months of tirzepatide treatment, there were significant reductions in total daily insulin [median (IQR) 73.9 (47.6-95.8) to 51.7 (46.7-66.8) units/day, p < 0.001], basal insulin [47 (28.2-51.8) to 32.4 (25.5-46.3) units/day, p < 0.001], and bolus insulin [31.4 (19.9-38.3) to 17.9 (14.9-22.2) units/day, p < 0.001] requirements. Insulin dose reduction from 2 to 8 months was modest. The frequency of user-initiated boluses did not differ throughout the study. Despite reductions in total insulin requirement, time in range (70-180 mg/dl) increased by 7%, A1c reduced by 0.5%, weight reduced by 9%, without increase in time below 70 mg/dl.
Conclusions: This pilot study provides clinical guidance on insulin titration for adults with T1D who may initiate tirzepatide therapy. Based on the findings of this study, we recommend reducing 25% of total daily insulin dose at tirzepatide initiation in adults with T1D using AID with baseline A1c of less than 7.5%. Higher doses of tirzepatide were associated with greater weight loss, however, the reduction in insulin requirement was minimal.
Keywords: Automated insulin delivery; HbA1c; Obesity; Tandem control IQ; Time in range; Tirzepatide; Type 1 diabetes; Weight loss.
© 2024. The Author(s).
Conflict of interest statement
The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Halis K. Akturk received research support through University of Colorado from Dexcom, Tandem Diabetes, Senseonics, Medtronic, Eli Lilly, REMD Biotherapeutics, IM Therapeutics, and IAFMS and received honoraria through University of Colorado from Senseonics and Mannkind for advisory board attendance. Viral N. Shah received research support from Novo Nordisk, Insulet, and Tandem Diabetes Care and received honoraria from Dexcom, Embecta, Insulet, Ascensia Diabetes Care, Tandem Diabetes Care, Genomelink and LumosFit for consulting or speaking arrangements. Kagan E. Karakus and Matthew P. Klien do not report any conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources

