Retinal Dystrophies Associated With Peripherin-2: Genetic Spectrum and Novel Clinical Observations in 241 Patients
- PMID: 38743414
- PMCID: PMC11098050
- DOI: 10.1167/iovs.65.5.22
Retinal Dystrophies Associated With Peripherin-2: Genetic Spectrum and Novel Clinical Observations in 241 Patients
Abstract
Purpose: To describe the clinical, electrophysiological and genetic spectrum of inherited retinal diseases associated with variants in the PRPH2 gene.
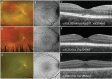
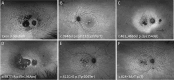
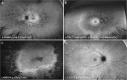
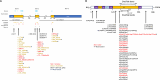
Methods: A total of 241 patients from 168 families across 15 sites in 9 countries with pathogenic or likely pathogenic variants in PRPH2 were included. Records were reviewed for age at symptom onset, visual acuity, full-field ERG, fundus colour photography, fundus autofluorescence (FAF), and SD-OCT. Images were graded into six phenotypes. Statistical analyses were performed to determine genotype-phenotype correlations.
Results: The median age at symptom onset was 40 years (range, 4-78 years). FAF phenotypes included normal (5%), butterfly pattern dystrophy, or vitelliform macular dystrophy (11%), central areolar choroidal dystrophy (28%), pseudo-Stargardt pattern dystrophy (41%), and retinitis pigmentosa (25%). Symptom onset was earlier in retinitis pigmentosa as compared with pseudo-Stargardt pattern dystrophy (34 vs 44 years; P = 0.004). The median visual acuity was 0.18 logMAR (interquartile range, 0-0.54 logMAR) and 0.18 logMAR (interquartile range 0-0.42 logMAR) in the right and left eyes, respectively. ERG showed a significantly reduced amplitude across all components (P < 0.001) and a peak time delay in the light-adapted 30-Hz flicker and single-flash b-wave (P < 0.001). Twenty-two variants were novel. The central areolar choroidal dystrophy phenotype was associated with 13 missense variants. The remaining variants showed marked phenotypic variability.
Conclusions: We described six distinct FAF phenotypes associated with variants in the PRPH2 gene. One FAF phenotype may have multiple ERG phenotypes, demonstrating a discordance between structure and function. Given the vast spectrum of PRPH2 disease our findings are useful for future clinical trials.
Conflict of interest statement
Disclosure:
Figures





References
-
- Boon CJ, den Hollander AI, Hoyng CB, Cremers FP, Klevering BJ, Keunen JE.. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retina Eye Res. 2008; 27(2): 213–235. - PubMed
-
- Michaelides M, Holder GE, Bradshaw K, Hunt DM, Moore AT.. Cone-rod dystrophy, intrafamilial variability, and incomplete penetrance associated with the R172W mutation in the peripherin/RDS gene. Ophthalmology. 2005; 112(9), 1592–1598. - PubMed

