Resistance to bacteriophage incurs a cost to virulence in drug-resistant Acinetobacter baumannii
- PMID: 38743467
- PMCID: PMC11170128
- DOI: 10.1099/jmm.0.001829
Resistance to bacteriophage incurs a cost to virulence in drug-resistant Acinetobacter baumannii
Abstract
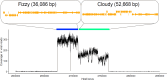
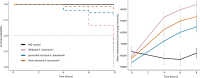
Introduction . Acinetobacter baumannii is a critical priority pathogen for novel antimicrobials (World Health Organization) because of the rise in nosocomial infections and its ability to evolve resistance to last resort antibiotics. A. baumannii is thus a priority target for phage therapeutics. Two strains of a novel, virulent bacteriophage (LemonAid and Tonic) able to infect carbapenem-resistant A. baumannii (strain NCTC 13420), were isolated from environmental water samples collected through a citizen science programme.Gap statement. Phage-host coevolution can lead to emergence of host resistance, with a concomitant reduction in the virulence of host bacteria; a potential benefit to phage therapy applications.Methodology. In vitro and in vivo assays, genomics and microscopy techniques were used to characterize the phages; determine mechanisms and impact of phage resistance on host virulence, and the efficacy of the phages against A. baumannii.Results. A. baumannii developed resistance to both viruses, LemonAid and Tonic. Resistance came at a cost to virulence, with the resistant variants causing significantly reduced mortality in a Galleria mellonella larval in vivo model. A replicated 8 bp insertion increased in frequency (~40 % higher frequency than in the wild-type) within phage-resistant A. baumannii mutants, putatively resulting in early truncation of a protein of unknown function. Evidence from comparative genomics and an adsorption assay suggests this protein acts as a novel phage receptor site in A. baumannii. We find no evidence linking resistance to changes in capsule structure, a known virulence factor. LemonAid efficiently suppressed growth of A. baumanni in vitro across a wide range of titres. However, in vivo, while survival of A. baumannii infected larvae significantly increased with both remedial and prophylactic treatment with LemonAid (107 p.f.u. ml-1), the effect was weak and not sufficient to save larvae from morbidity and mortality.Conclusion. While LemonAid and Tonic did not prove effective as a treatment in a Galleria larvae model, there is potential to harness their ability to attenuate virulence in drug-resistant A. baumannii.
Keywords: Acinetobacter baumannii; Galleria mellonella; lytic; phage resistance; virulence.
Conflict of interest statement
There are no known conflicts of interest.
Figures




References
-
- Public Health England N. Detection of bacteria with carbapenem-hydrolysing betat-lactamases (carbapenemases) 2022.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

