Fibulin-2 is an extracellular matrix inhibitor of oligodendrocytes relevant to multiple sclerosis
- PMID: 38743490
- PMCID: PMC11213512
- DOI: 10.1172/JCI176910
Fibulin-2 is an extracellular matrix inhibitor of oligodendrocytes relevant to multiple sclerosis
Abstract
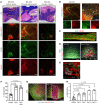
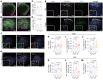
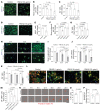
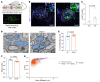
Impairment of oligodendrocytes and myelin contributes to neurological disorders including multiple sclerosis (MS), stroke, and Alzheimer's disease. Regeneration of myelin (remyelination) decreases the vulnerability of demyelinated axons, but this repair process commonly fails with disease progression. A contributor to inefficient remyelination is the altered extracellular matrix (ECM) in lesions, which remains to be better defined. We have identified fibulin-2 (FBLN2) as a highly upregulated ECM component in lesions of MS and stroke and in proteome databases of Alzheimer's disease and traumatic brain injury. Focusing on MS, the inhibitory role of FBLN2 was suggested in the experimental autoimmune encephalomyelitis (EAE) model, in which genetic FBLN2 deficiency improved behavioral recovery by promoting the maturation of oligodendrocytes and enhancing remyelination. Mechanistically, when oligodendrocyte progenitors were cultured in differentiation medium, FBLN2 impeded their maturation into oligodendrocytes by engaging the Notch pathway, leading to cell death. Adeno-associated virus deletion of FBLN2 in astrocytes improved oligodendrocyte numbers and functional recovery in EAE and generated new myelin profiles after lysolecithin-induced demyelination. Collectively, our findings implicate FBLN2 as a hitherto unrecognized injury-elevated ECM, and a therapeutic target, that impairs oligodendrocyte maturation and myelin repair.
Keywords: Extracellular matrix; Multiple sclerosis; Neuroscience.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

