ZBP1-mediated apoptosis and inflammation exacerbate steatotic liver ischemia/reperfusion injury
- PMID: 38743492
- PMCID: PMC11213514
- DOI: 10.1172/JCI180451
ZBP1-mediated apoptosis and inflammation exacerbate steatotic liver ischemia/reperfusion injury
Abstract
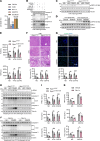
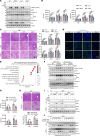
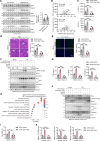
Steatotic donor livers are becoming more and more common in liver transplantation. However, the current use of steatotic grafts is less acceptable than normal grafts due to their higher susceptibility to ischemia/reperfusion (I/R) injury. To investigate the mechanism underlying the susceptibility of steatotic liver to I/R injury, we detected cell death markers and inflammation in clinical donor livers and animal models. We found that caspase-8-mediated hepatic apoptosis is activated in steatotic liver I/R injury. However, ablation of caspase-8 only slightly mitigated steatotic liver I/R injury without affecting inflammation. We further demonstrated that RIPK1 kinase induces both caspase-8-mediated apoptosis and cell death-independent inflammation. Inhibition of RIPK1 kinase significantly protects against steatotic liver I/R injury by alleviating both hepatic apoptosis and inflammation. Additionally, we found that RIPK1 activation is induced by Z-DNA binding protein 1 (ZBP1) but not the canonical TNF-α pathway during steatotic liver I/R injury. Deletion of ZBP1 substantially decreases the steatotic liver I/R injury. Mechanistically, ZBP1 is amplified by palmitic acid-activated JNK pathway in steatotic livers. Upon I/R injury, excessive reactive oxygen species trigger ZBP1 activation by inducing its aggregation independent of the Z-nucleic acids sensing action in steatotic livers, leading to the kinase activation of RIPK1 and the subsequent aggravation of liver injury. Thus, ZBP1-mediated RIPK1-driven apoptosis and inflammation exacerbate steatotic liver I/R injury, which could be targeted to protect steatotic donor livers during transplantation.
Keywords: Apoptosis; Transplantation.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

