PMP22 duplication dysregulates lipid homeostasis and plasma membrane organization in developing human Schwann cells
- PMID: 38743588
- PMCID: PMC11370802
- DOI: 10.1093/brain/awae158
PMP22 duplication dysregulates lipid homeostasis and plasma membrane organization in developing human Schwann cells
Abstract
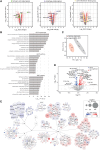
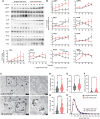
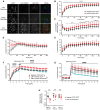
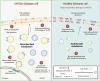
Charcot-Marie-Tooth disease type 1A (CMT1A) is the most common inherited peripheral neuropathy caused by a 1.5 Mb tandem duplication of chromosome 17 harbouring the PMP22 gene. This dose-dependent overexpression of PMP22 results in disrupted Schwann cell myelination of peripheral nerves. To obtain better insights into the underlying pathogenic mechanisms in CMT1A, we investigated the role of PMP22 duplication in cellular homeostasis in CMT1A mouse models and in patient-derived induced pluripotent stem cells differentiated into Schwann cell precursors (iPSC-SCPs). We performed lipidomic profiling and bulk RNA sequencing (RNA-seq) on sciatic nerves of two developing CMT1A mouse models and on CMT1A patient-derived iPSC-SCPs. For the sciatic nerves of the CMT1A mice, cholesterol and lipid metabolism was downregulated in a dose-dependent manner throughout development. For the CMT1A iPSC-SCPs, transcriptional analysis unveiled a strong suppression of genes related to autophagy and lipid metabolism. Gene ontology enrichment analysis identified disturbances in pathways related to plasma membrane components and cell receptor signalling. Lipidomic analysis confirmed the severe dysregulation in plasma membrane lipids, particularly sphingolipids, in CMT1A iPSC-SCPs. Furthermore, we identified reduced lipid raft dynamics, disturbed plasma membrane fluidity and impaired cholesterol incorporation and storage, all of which could result from altered lipid storage homeostasis in the patient-derived CMT1A iPSC-SCPs. Importantly, this phenotype could be rescued by stimulating autophagy and lipolysis. We conclude that PMP22 duplication disturbs intracellular lipid storage and leads to a more disordered plasma membrane owing to an alteration in the lipid composition, which might ultimately lead to impaired axo-glial interactions. Moreover, targeting lipid handling and metabolism could hold promise for the treatment of patients with CMT1A.
Keywords: Charcot–Marie–Tooth disease type 1A; Schwann cells; human induced pluripotent stem cells; lipid metabolism; lipid storage; plasma membrane.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
L.V.D.B. is head of the Scientific Advisory Board of Augustine Therapeutics (Leuven, Belgium) and is part of the Investment Advisory Board of Droia Ventures (Meise, Belgium). The other authors declare no competing interests.
Figures




References
-
- Timmerman V, Neils E, Van Hul W, et al. The peripheral myelin protein gene PMP-22 is contained within the Charcot-Marie-Tooth disease type 1A duplication. Nat Genet. 1992;1:171–175. - PubMed
-
- Raeymaekers P, Timmerman V, Nelis E, et al. Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1a (CMT 1a). Neuromuscul Disord. 1991;1:93–97. - PubMed
-
- Amici SA, Dunn WA, Notterpek L. Developmental abnormalities in the nerves of peripheral myelin protein 22-deficient mice. J Neurosci Res. 2007;85:238–249. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

