Copper-Nitroxyl-Catalyzed α-Oxygenation of Cyclic Secondary Amines Including Application to Late-Stage Functionalization
- PMID: 38743876
- PMCID: PMC11409824
- DOI: 10.1021/jacs.4c04359
Copper-Nitroxyl-Catalyzed α-Oxygenation of Cyclic Secondary Amines Including Application to Late-Stage Functionalization
Abstract
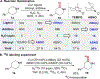
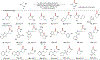
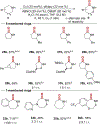
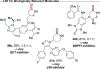
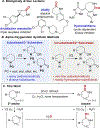
Cyclic secondary amines are prominent subunits in pharmaceutical compounds. Methods for direct functionalization of N-unprotected/unsubstituted piperidines and related heterocycles have limited precedent despite their potential to impact medicinal chemistry and organic synthesis. Herein, we report a Cu/nitroxyl co-catalyzed method for direct conversion of cyclic secondary amines to the corresponding lactams via aerobic dehydrogenation and oxidative coupling with water. The mild reaction conditions tolerate diverse functional groups, enabling application to molecules that cover broad chemical space. The method is showcased in selective functionalization of building blocks and complex molecules, including late-stage functionalization of bromodomain inhibitors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
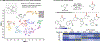

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

