Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models
- PMID: 38743922
- PMCID: PMC11290972
- DOI: 10.1172/JCI175616
Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models
Abstract
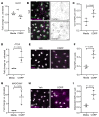
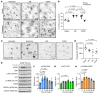
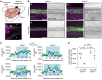
Recently developed antimigraine therapeutics targeting calcitonin gene-related peptide (CGRP) signaling are effective, though their sites of activity remain elusive. Notably, the lymphatic vasculature is responsive to CGRP signaling, but whether meningeal lymphatic vessels (MLVs) contribute to migraine pathophysiology is unknown. Mice with lymphatic vasculature deficient in the CGRP receptor (CalcrliLEC mice) treated with nitroglycerin-mediated (NTG-mediated) chronic migraine exhibit reduced pain and light avoidance compared with NTG-treated littermate controls. Gene expression profiles of lymphatic endothelial cells (LECs) isolated from the meninges of Rpl22HA/+;Lyve1Cre RiboTag mice treated with NTG revealed increased MLV-immune interactions compared with cells from untreated mice. Interestingly, the relative abundance of mucosal vascular addressin cell adhesion molecule 1-interacting (MAdCAM1-interacting) CD4+ T cells was increased in the deep cervical lymph nodes of NTG-treated control mice but not in NTG-treated CalcrliLEC mice. Treatment of cultured hLECs with CGRP peptide in vitro induced vascular endothelial-cadherin (VE-cadherin) rearrangement and reduced functional permeability. Likewise, intra cisterna magna injection of CGRP caused rearrangement of VE-cadherin, decreased MLV uptake of cerebrospinal fluid (CSF), and impaired CSF drainage in control mice but not in CalcrliLEC mice. Collectively, these findings reveal a previously unrecognized role for lymphatics in chronic migraine, whereby CGRP signaling primes MLV-immune interactions and reduces CSF efflux.
Keywords: Cell biology; G protein–coupled receptors; Lymph; Pain; Vascular biology.
Conflict of interest statement
Figures






Comment in
-
Meningeal lymphatic vessel dysfunction driven by CGRP signaling causes migraine-like pain in mice.J Clin Invest. 2024 Aug 1;134(15):e182556. doi: 10.1172/JCI182556. J Clin Invest. 2024. PMID: 39087472 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

