An investigation of the clinical impact and therapeutic relevance of a DNA damage immune response (DDIR) signature in patients with advanced gastroesophageal adenocarcinoma
- PMID: 38744099
- PMCID: PMC11108838
- DOI: 10.1016/j.esmoop.2024.103450
An investigation of the clinical impact and therapeutic relevance of a DNA damage immune response (DDIR) signature in patients with advanced gastroesophageal adenocarcinoma
Abstract
Background: An improved understanding of which gastroesophageal adenocarcinoma (GOA) patients respond to both chemotherapy and immune checkpoint inhibitors (ICI) is needed. We investigated the predictive role and underlying biology of a 44-gene DNA damage immune response (DDIR) signature in patients with advanced GOA.
Materials and methods: Transcriptional profiling was carried out on pretreatment tissue from 252 GOA patients treated with platinum-based chemotherapy (three dose levels) within the randomized phase III GO2 trial. Cross-validation was carried out in two independent GOA cohorts with transcriptional profiling, immune cell immunohistochemistry and epidermal growth factor receptor (EGFR) fluorescent in situ hybridization (FISH) (n = 430).
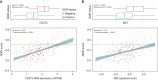
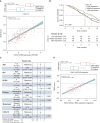
Results: In the GO2 trial, DDIR-positive tumours had a greater radiological response (51.7% versus 28.5%, P = 0.022) and improved overall survival in a dose-dependent manner (P = 0.028). DDIR positivity was associated with a pretreatment inflamed tumour microenvironment (TME) and increased expression of biomarkers associated with ICI response such as CD274 (programmed death-ligand 1, PD-L1) and a microsatellite instability RNA signature. Consensus pathway analysis identified EGFR as a potential key determinant of the DDIR signature. EGFR amplification was associated with DDIR negativity and an immune cold TME.
Conclusions: Our results indicate the importance of the GOA TME in chemotherapy response, its relationship to DNA damage repair and EGFR as a targetable driver of an immune cold TME. Chemotherapy-sensitive inflamed GOAs could benefit from ICI delivered in combination with standard chemotherapy. Combining EGFR inhibitors and ICIs warrants further investigation in patients with EGFR-amplified tumours.
Keywords: DNA damage immune response; epidermal growth factor receptor; gastroesophageal adenocarcinoma; immune checkpoint inhibitors; tumour microenvironment.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure MAB reported personal fees from Ipsen, BMS and Servier. RDP reported personal fees from Eli Lilly, Bristol Myers Squib, and Servier and grants from AstraZeneca, Roche, Sanofi, Merck Sharp & Dohme, Five Prime Therapeutics and Jansen outside the submitted work. MJS reported grants from Cancer Research UK during the conduct of the GO2 study. PSH reported grants from Cancer Research UK during the conduct of the study and institutional research funding from Novartis, Pfizer, Eli Lilly, Daiichi-Sankyo and Eisai outside the submitted work. GL and RDK are employees of Almac Diagnostic Services. RT reported personal fees from Eli Lilly, Astellas and Almac Diagnostic Services outside the submitted work. All other authors have declared no conflicts of interest.
Figures


References
-
- Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Healthcare Quality Improvement Partnership . 2019. National Oesophago-Gastric Cancer Audit – an audit of the care received by people with oesophago-gastric cancer and oesophageal high grade dysplasia in England and Wales.
-
- Cunningham D., Starling N., Rao S., et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. - PubMed
-
- Hall P.S., Swinson D., Cairns D.A., et al. Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):869–877. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

