The adipose-neural axis is involved in epicardial adipose tissue-related cardiac arrhythmias
- PMID: 38744275
- PMCID: PMC11148799
- DOI: 10.1016/j.xcrm.2024.101559
The adipose-neural axis is involved in epicardial adipose tissue-related cardiac arrhythmias
Abstract
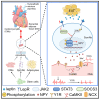
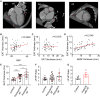
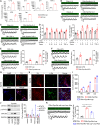
Dysfunction of the sympathetic nervous system and increased epicardial adipose tissue (EAT) have been independently associated with the occurrence of cardiac arrhythmia. However, their exact roles in triggering arrhythmia remain elusive. Here, using an in vitro coculture system with sympathetic neurons, cardiomyocytes, and adipocytes, we show that adipocyte-derived leptin activates sympathetic neurons and increases the release of neuropeptide Y (NPY), which in turn triggers arrhythmia in cardiomyocytes by interacting with the Y1 receptor (Y1R) and subsequently enhancing the activity of the Na+/Ca2+ exchanger (NCX) and calcium/calmodulin-dependent protein kinase II (CaMKII). The arrhythmic phenotype can be partially blocked by a leptin neutralizing antibody or an inhibitor of Y1R, NCX, or CaMKII. Moreover, increased EAT thickness and leptin/NPY blood levels are detected in atrial fibrillation patients compared with the control group. Our study provides robust evidence that the adipose-neural axis contributes to arrhythmogenesis and represents a potential target for treating arrhythmia.
Keywords: NPY; adipose-neural axis; arrhythmia; epicardial adipose tissue; leptin; sympathetic neurons.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Thomas D., Christ T., Fabritz L., Goette A., Hammwöhner M., Heijman J., Kockskämper J., Linz D., Odening K.E., Schweizer P.A., et al. German Cardiac Society Working Group on Cellular Electrophysiology state-of-the-art paper: impact of molecular mechanisms on clinical arrhythmia management. Clin. Res. Cardiol. 2019;108:577–599. doi: 10.1007/s00392-018-1377-1. - DOI - PubMed
-
- Wong C.X., Sun M.T., Odutayo A., Emdin C.A., Mahajan R., Lau D.H., Pathak R.K., Wong D.T., Selvanayagam J.B., Sanders P., Clarke R. Associations of Epicardial, Abdominal, and Overall Adiposity With Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004378. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

