Vertebrate centromeres in mitosis are functionally bipartite structures stabilized by cohesin
- PMID: 38744280
- PMCID: PMC11164432
- DOI: 10.1016/j.cell.2024.04.014
Vertebrate centromeres in mitosis are functionally bipartite structures stabilized by cohesin
Abstract
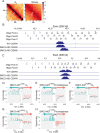
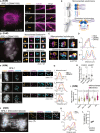
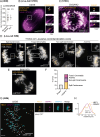
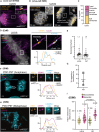
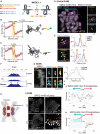
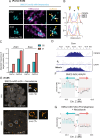
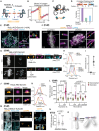
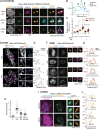
Centromeres are scaffolds for the assembly of kinetochores that ensure chromosome segregation during cell division. How vertebrate centromeres obtain a three-dimensional structure to accomplish their primary function is unclear. Using super-resolution imaging, capture-C, and polymer modeling, we show that vertebrate centromeres are partitioned by condensins into two subdomains during mitosis. The bipartite structure is found in human, mouse, and chicken cells and is therefore a fundamental feature of vertebrate centromeres. Super-resolution imaging and electron tomography reveal that bipartite centromeres assemble bipartite kinetochores, with each subdomain binding a distinct microtubule bundle. Cohesin links the centromere subdomains, limiting their separation in response to spindle forces and avoiding merotelic kinetochore-spindle attachments. Lagging chromosomes during cancer cell divisions frequently have merotelic attachments in which the centromere subdomains are separated and bioriented. Our work reveals a fundamental aspect of vertebrate centromere biology with implications for understanding the mechanisms that guarantee faithful chromosome segregation.
Keywords: centromere; chromatin organization; chromosomal instability; cohesin; condensin; kinetochore; mitosis.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

