End of 2022/23 Season Influenza Vaccine Effectiveness in Primary Care in Great Britain
- PMID: 38744684
- PMCID: PMC11093773
- DOI: 10.1111/irv.13295
End of 2022/23 Season Influenza Vaccine Effectiveness in Primary Care in Great Britain
Abstract
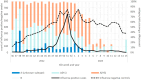
Background: The 2022/23 influenza season in the United Kingdom saw the return of influenza to prepandemic levels following two seasons with low influenza activity. The early season was dominated by A(H3N2), with cocirculation of A(H1N1), reaching a peak late December 2022, while influenza B circulated at low levels during the latter part of the season. From September to March 2022/23, influenza vaccines were offered, free of charge, to all aged 2-13 (and 14-15 in Scotland and Wales), adults up to 49 years of age with clinical risk conditions and adults aged 50 and above across the mainland United Kingdom.
Methods: End-of-season adjusted vaccine effectiveness (VE) estimates against sentinel primary-care attendance for influenza-like illness, where influenza infection was laboratory confirmed, were calculated using the test negative design, adjusting for potential confounders.
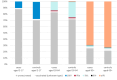
Methods: Results In the mainland United Kingdom, end-of-season VE against all laboratory-confirmed influenza for all those > 65 years of age, most of whom received adjuvanted quadrivalent vaccines, was 30% (95% CI: -6% to 54%). VE for those aged 18-64, who largely received cell-based vaccines, was 47% (95% CI: 37%-56%). Overall VE for 2-17 year olds, predominantly receiving live attenuated vaccines, was 66% (95% CI: 53%-76%).
Conclusion: The paper provides evidence of moderate influenza VE in 2022/23.
Keywords: effectiveness; influenza; vaccine.
© 2024 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Conflict of interest statement
SdeL, within his academic role, is the director of the RCGP RSC. He has received grants through his University from AstraZeneca, GSK, Moderna, Sanofi and Seqirus for vaccine‐related research and been a member of advisory boards for AstraZeneca, GSK, Sanofi and Seqirus. HW and CHW's department has received cost‐recovery payment from CSL Seqirus for analysis undertaken for regulatory review.
Figures

References
-
- UK Health Security Agency , “Surveillance of Influenza and Other Seasonal Respiratory Viruses in Winter 2021 to 2022,” UK Health Security Agency, 30/09/2022, (2022), https://www.gov.uk/government/statistics/annual‐flu‐reports/surveillance....
-
- Public Health England , “Surveillance of Influenza and Other Seasonal Respiratory Viruses in the UK: Winter 2020 to 2021,” (2021), https://webarchive.nationalarchives.gov.uk/ukgwa/20220401215804mp_/https....
-
- UK Health Security Agency , “Surveillance of Influenza and Other Seasonal Respiratory Viruses in the UK, Winter 2022 to 2023,” (2023), https://www.gov.uk/government/statistics/annual‐flu‐reports/surveillance....
-
- Pebody R., Whitaker H., Zhao H., et al., “Protection Provided by Influenza Vaccine Against Influenza‐Related Hospitalisation in >/=65 Year Olds: Early Experience of Introduction of a Newly Licensed Adjuvanted Vaccine in England in 2018/19,” Vaccine 38, no. 2 (2020): 173–179. - PubMed
-
- Department of Health and Social Care , “National Flu Immunisation Programme 2022 to 2023 Letter,” (2022), https://webarchive.nationalarchives.gov.uk/ukgwa/20230515154700/https://....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

