2-APQC, a small-molecule activator of Sirtuin-3 (SIRT3), alleviates myocardial hypertrophy and fibrosis by regulating mitochondrial homeostasis
- PMID: 38744811
- PMCID: PMC11094072
- DOI: 10.1038/s41392-024-01816-1
2-APQC, a small-molecule activator of Sirtuin-3 (SIRT3), alleviates myocardial hypertrophy and fibrosis by regulating mitochondrial homeostasis
Abstract
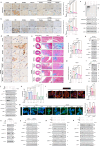
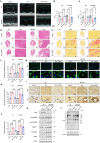
Sirtuin 3 (SIRT3) is well known as a conserved nicotinamide adenine dinucleotide+ (NAD+)-dependent deacetylase located in the mitochondria that may regulate oxidative stress, catabolism and ATP production. Accumulating evidence has recently revealed that SIRT3 plays its critical roles in cardiac fibrosis, myocardial fibrosis and even heart failure (HF), through its deacetylation modifications. Accordingly, discovery of SIRT3 activators and elucidating their underlying mechanisms of HF should be urgently needed. Herein, we identified a new small-molecule activator of SIRT3 (named 2-APQC) by the structure-based drug designing strategy. 2-APQC was shown to alleviate isoproterenol (ISO)-induced cardiac hypertrophy and myocardial fibrosis in vitro and in vivo rat models. Importantly, in SIRT3 knockout mice, 2-APQC could not relieve HF, suggesting that 2-APQC is dependent on SIRT3 for its protective role. Mechanically, 2-APQC was found to inhibit the mammalian target of rapamycin (mTOR)-p70 ribosomal protein S6 kinase (p70S6K), c-jun N-terminal kinase (JNK) and transforming growth factor-β (TGF-β)/ small mother against decapentaplegic 3 (Smad3) pathways to improve ISO-induced cardiac hypertrophy and myocardial fibrosis. Based upon RNA-seq analyses, we demonstrated that SIRT3-pyrroline-5-carboxylate reductase 1 (PYCR1) axis was closely assoiated with HF. By activating PYCR1, 2-APQC was shown to enhance mitochondrial proline metabolism, inhibited reactive oxygen species (ROS)-p38 mitogen activated protein kinase (p38MAPK) pathway and thereby protecting against ISO-induced mitochondrialoxidative damage. Moreover, activation of SIRT3 by 2-APQC could facilitate AMP-activated protein kinase (AMPK)-Parkin axis to inhibit ISO-induced necrosis. Together, our results demonstrate that 2-APQC is a targeted SIRT3 activator that alleviates myocardial hypertrophy and fibrosis by regulating mitochondrial homeostasis, which may provide a new clue on exploiting a promising drug candidate for the future HF therapeutics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. Liang Ouyang is the editorial board member of Signal Transduction and Targeted Therapy, but he has not been involved in the process of the paper handling.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 82374020/National Natural Science Foundation of China (National Science Foundation of China)
- 82073998/National Natural Science Foundation of China (National Science Foundation of China)
- 22107015/National Natural Science Foundation of China (National Science Foundation of China)
- 82173666/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

