Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: pooled phase 1, 2, 3a and 3b randomized, controlled trials
- PMID: 38744844
- PMCID: PMC11094049
- DOI: 10.1038/s41467-024-47905-1
Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: pooled phase 1, 2, 3a and 3b randomized, controlled trials
Abstract
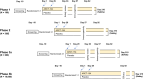
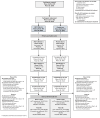
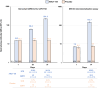
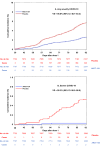
Combination of waning immunity and lower effectiveness against new SARS-CoV-2 variants of approved COVID-19 vaccines necessitates new vaccines. We evaluated two doses, 28 days apart, of ARCT-154, a self-amplifying mRNA COVID-19 vaccine, compared with saline placebo in an integrated phase 1/2/3a/3b controlled, observer-blind trial in Vietnamese adults (ClinicalTrial.gov identifier: NCT05012943). Primary safety and reactogenicity outcomes were unsolicited adverse events (AE) 28 days after each dose, solicited local and systemic AE 7 days after each dose, and serious AEs throughout the study. Primary immunogenicity outcome was the immune response as neutralizing antibodies 28 days after the second dose. Efficacy against COVID-19 was assessed as primary and secondary outcomes in phase 3b. ARCT-154 was well tolerated with generally mild-moderate transient AEs. Four weeks after the second dose 94.1% (95% CI: 92.1-95.8) of vaccinees seroconverted for neutralizing antibodies, with a geometric mean-fold rise from baseline of 14.5 (95% CI: 13.6-15.5). Of 640 cases of confirmed COVID-19 eligible for efficacy analysis most were due to the Delta (B.1.617.2) variant. Efficacy of ARCT-154 was 56.6% (95% CI: 48.7- 63.3) against any COVID-19, and 95.3% (80.5-98.9) against severe COVID-19. ARCT-154 vaccination is well tolerated, immunogenic and efficacious, particularly against severe COVID-19 disease.
© 2024. The Author(s).
Conflict of interest statement
S.H., S.P., B.S., S.S., Q.R., B.C., B.L., K.L., D.B., K.M., R.S., B.G. and I.S. were all full-time employees of the vaccine manufacturer and study sponsor, Arcturus Therapeutics, Inc., at the time of the study. T.T.L.L. and N.T.V. are employees of the vaccine licensee, Vietnam Biocare Biotechnology Joint Stock Company. Other authors declare no competing interests.
Figures


References
-
- Lenharo M. WHO declares end to COVID-19's emergency phase. Nature10.1038/d41586-023-01559-z (2023). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

