Transcription stress at telomeres leads to cytosolic DNA release and paracrine senescence
- PMID: 38744897
- PMCID: PMC11094137
- DOI: 10.1038/s41467-024-48443-6
Transcription stress at telomeres leads to cytosolic DNA release and paracrine senescence
Abstract
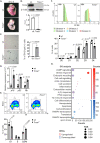
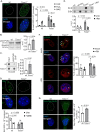
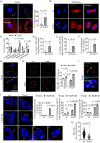
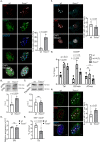
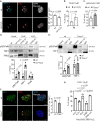
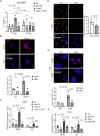
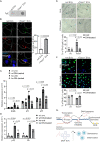
Transcription stress has been linked to DNA damage -driven aging, yet the underlying mechanism remains unclear. Here, we demonstrate that Tcea1-/- cells, which harbor a TFIIS defect in transcription elongation, exhibit RNAPII stalling at oxidative DNA damage sites, impaired transcription, accumulation of R-loops, telomere uncapping, chromatin bridges, and genome instability, ultimately resulting in cellular senescence. We found that R-loops at telomeres causally contribute to the release of telomeric DNA fragments in the cytoplasm of Tcea1-/- cells and primary cells derived from naturally aged animals triggering a viral-like immune response. TFIIS-defective cells release extracellular vesicles laden with telomeric DNA fragments that target neighboring cells, which consequently undergo cellular senescence. Thus, transcription stress elicits paracrine signals leading to cellular senescence, promoting aging.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

