Multifunctional in vitro, in silico and DFT analyses on antimicrobial BagremycinA biosynthesized by Micromonospora chokoriensis CR3 from Hieracium canadense
- PMID: 38745055
- PMCID: PMC11093986
- DOI: 10.1038/s41598-024-61490-9
Multifunctional in vitro, in silico and DFT analyses on antimicrobial BagremycinA biosynthesized by Micromonospora chokoriensis CR3 from Hieracium canadense
Abstract
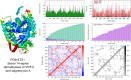
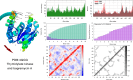
Among the actinomycetes in the rare genera, Micromonospora is of great interest since it has been shown to produce novel therapeutic compounds. Particular emphasis is now on its isolation from plants since its population from soil has been extensively explored. The strain CR3 was isolated as an endophyte from the roots of Hieracium canadense, and it was identified as Micromonospora chokoriensis through 16S gene sequencing and phylogenetic analysis. The in-vitro analysis of its extract revealed it to be active against the clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) and Candida tropicalis (15 mm). No bioactivity was observed against Gram-negative bacteria, Escherichia coli ATCC 25922, and Klebsiella pneumoniae ATCC 706003. The Micromonospora chokoriensis CR3 extract was also analyzed through the HPLC-DAD-UV-VIS resident database, and it gave a maximum match factor of 997.334 with the specialized metabolite BagremycinA (BagA). The in-silico analysis indicated that BagA strongly interacted with the active site residues of the sterol 14-α demethylase and thymidylate kinase enzymes, with the lowest binding energies of - 9.7 and - 8.3 kcal/mol, respectively. Furthermore, the normal mode analysis indicated that the interaction between these proteins and BagA was stable. The DFT quantum chemical properties depicted BagA to be reasonably reactive with a HOMO-LUMO gap of (ΔE) of 4.390 eV. BagA also passed the drug-likeness test with a synthetic accessibility score of 2.06, whereas Protox-II classified it as a class V toxicity compound with high LD50 of 2644 mg/kg. The current study reports an endophytic actinomycete, M. chokoriensis, associated with H. canadense producing the bioactive metabolite BagA with promising antimicrobial activity, which can be further modified and developed into a safe antimicrobial drug.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




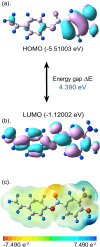
Similar articles
-
Rare actinomycetes Nocardia caishijiensis and Pseudonocardia carboxydivorans as endophytes, their bioactivity and metabolites evaluation.Microbiol Res. 2016 Apr;185:22-35. doi: 10.1016/j.micres.2016.01.003. Epub 2016 Jan 23. Microbiol Res. 2016. PMID: 26946375
-
Metabolomic and molecular insights into adenosine triphosphate synthase inhibitors from Micromonospora sp. as novel antimicrobial agents against multidrug-resistant Gram-negative pathogens of enteric origin.Lett Appl Microbiol. 2025 May 1;78(5):ovaf064. doi: 10.1093/lambio/ovaf064. Lett Appl Microbiol. 2025. PMID: 40275498
-
In vitro antimicrobial and antioxidant activities of bioactive compounds extracted from Streptomyces africanus strain E2 isolated from Moroccan soil.Sci Rep. 2024 Nov 9;14(1):27372. doi: 10.1038/s41598-024-77729-4. Sci Rep. 2024. PMID: 39521814 Free PMC article.
-
Benzothiazole Clubbed Imidazolone Derivatives: Synthesis, Molecular Docking, DFT Studies, and Antimicrobial Studies.Curr Comput Aided Drug Des. 2023;19(2):123-136. doi: 10.2174/1573409919666221121115556. Curr Comput Aided Drug Des. 2023. PMID: 36411562
-
Further Polycyclic Quinones of Micromonospora sp.Chem Biodivers. 2024 Jul;21(7):e202301771. doi: 10.1002/cbdv.202301771. Epub 2024 May 31. Chem Biodivers. 2024. PMID: 38628065 Review.
Cited by
-
Gallic Acid: A Potent Metabolite Targeting Shikimate Kinase in Acinetobacter baumannii.Metabolites. 2024 Dec 23;14(12):727. doi: 10.3390/metabo14120727. Metabolites. 2024. PMID: 39728508 Free PMC article.
References
-
- Mrid RB, Benmrid B, Hafsa J, Boukcim H, Sobeh M, Yasri A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021;777:146204. doi: 10.1016/j.scitotenv.2021.146204. - DOI
-
- Ezeobiora CE, et al. Uncovering the biodiversity and biosynthetic potentials of rare actinomycetes. Future J. Pharm. Sci. 2022;8:1–9.
-
- Ahmed W, Helal IM, Zaky MM, Abdulla HM. Antimicrobial activity of actinomycetes extracts against multidrug-resistant Staphylococcus aureus and Salmonella spp. isolated from meat. Alfarama J. Basic Appl. Sci. 2022;3:283–99.
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

