Neutrophil-macrophage communication via extracellular vesicle transfer promotes itaconate accumulation and ameliorates cytokine storm syndrome
- PMID: 38745069
- PMCID: PMC11637192
- DOI: 10.1038/s41423-024-01174-6
Neutrophil-macrophage communication via extracellular vesicle transfer promotes itaconate accumulation and ameliorates cytokine storm syndrome
Abstract
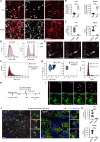
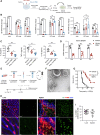
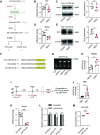
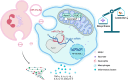
Cytokine storm syndrome (CSS) is a life-threatening systemic inflammatory syndrome involving innate immune hyperactivity triggered by various therapies, infections, and autoimmune conditions. However, the potential interplay between innate immune cells is not fully understood. Here, using poly I:C and lipopolysaccharide (LPS)-induced cytokine storm models, a protective role of neutrophils through the modulation of macrophage activation was identified in a CSS model. Intravital imaging revealed neutrophil-derived extracellular vesicles (NDEVs) in the liver and spleen, which were captured by macrophages. NDEVs suppressed proinflammatory cytokine production by macrophages when cocultured in vitro or infused into CSS models. Metabolic profiling of macrophages treated with NDEV revealed elevated levels of the anti-inflammatory metabolite, itaconate, which is produced from cis-aconitate in the Krebs cycle by cis-aconitate decarboxylase (Acod1, encoded by Irg1). Irg1 in macrophages, but not in neutrophils, was critical for the NDEV-mediated anti-inflammatory effects. Mechanistically, NDEVs delivered miR-27a-3p, which suppressed the expression of Suclg1, the gene encoding the enzyme that metabolizes itaconate, thereby resulting in the accumulation of itaconate in macrophages. These findings demonstrated that neutrophil-to-macrophage communication mediated by extracellular vesicles is critical for promoting the anti-inflammatory reprogramming of macrophages in CSS and may have potential implications for the treatment of this fatal condition.
Keywords: Cytokine storm syndrome; Extracellular vesicle; Itaconate; Macrophage; Neutrophil.
© 2024. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ryan DG, O’Neill LAJ. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38:289–313. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

